Asclepias humistrata
| Asclepias humistrata | |
|---|---|

| |
| Asclepias humistrata | |
| Scientific classification | |
| Kingdom: | Plantae |
| Division: | Magnoliophyta - Flowering plants |
| Class: | Dicots |
| Order: | Gentianales |
| Family: | Apocynaceae |
| Genus: | Asclepias |
| Species: | A. humistrata |
| Binomial name | |
| Asclepias humistrata Walter | |
| |
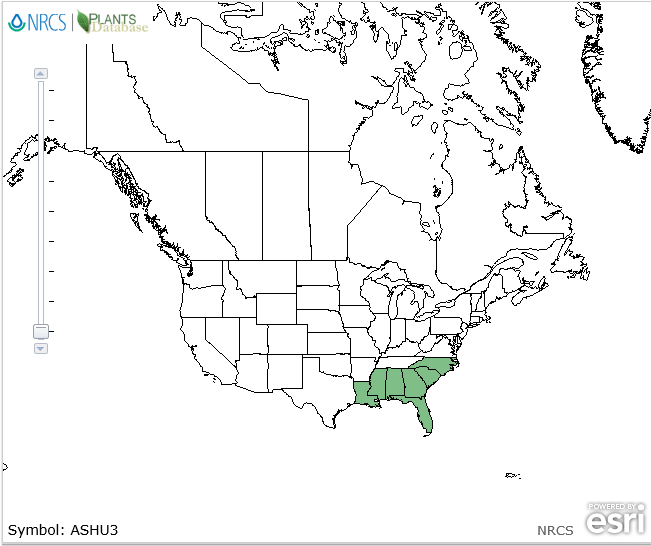
| Natural range of Asclepias humistrata from USDA NRCS [1]. | |
Common names: Pinewoods milkweed; Sandhill milkweed
Contents
Taxonomic notes
Asclepias is named for Asklepio, the Greek god of medicine and healing. Humistrata is derived from the Latin word 'humis' meaning ground and 'sternere' to spread.[1]
Synonyms: none.[2]
Varieties: none.[2]
Description
In general, with the Asclepias genus, these plants are perennial herbs containing milky sap. The stems are erect, spreading or decumbent and usually are simple and often solitary. The leaves are opposite to subopposite, are sometimes whorled, and rarely alternate. The corolla lobes are reflexed and are rarely erect or spreading. The filaments are elaborate five hoods forming a corona around the gynosteguim. The corona horns are present in most species.[3]
More specifically, for A. humistrata, the stems are glabrous, simple, stout, and rarely solitary; they spread ascendingly, and grow 20 - 70 cm tall. The leaves are opposite, about 5 - 8 pairs, ovate in shape, 6 - 10 cm long and 4.5 - 8.5 cm wide. The leaves are widely acute to obtuse, the margins are flat, auriculate, more or less amplexicaul, subsucculent, glaucous, the veins are pink to lavender in color, and are sessile. There are 2 - 5 or more umbels beginning from the upper 2 - 5 nodes, and are 3 - 5 cm wide. The flower is pale rose or lavender in color, the lobes are reflexed, and are 5 - 6.5 mm long. The corona is 3 - 5 mm in diameter. The horns are shorter than the hood. The follicles are erect and are 9 - 14 cm long, 1.3 - 1.8 cm broad.[3]
The root system of Asclepias humistrata includes stem tubers which store non-structural carbohydrates (NSC) important for both resprouting following fire and persisting during long periods of fire exclusion.[4] Diaz-Toribio and Putz (2021) recorded this species to have an NSC concentration of 171 mg/g (ranking 28 out of 100 species studied) and water content of 62.4% (ranking 55 out of 100 species studied).[4]
According to Diaz-Torbio and Putz (2021), Asclepais humistrata has stem tubers with a below-ground to above-ground biomass ratio of 1.205 and nonstructural carbohydrate concentration of 171 mg g-1.[5]
Distribution
Endemic to the southeastern U.S.: North Carolina south to Florida and west to Louisiana.[1]
Ecology
Habitat
In the Coastal Plain in Florida and Georgia, A. humistrata can occur in scrub oak sand ridges, longleaf pine-scrub oak ridges, pine-palmetto thickets, turkey oak scrubs, low sand dunes, and mixed pine hardwood associations. It can occur in disturbed areas such as sandy fallow fields and roadsides. Soil types include loamy sand and coarse sand.[6]
Associated species include Coreopsis basalis, Hymenopappus scabiosaeus, Liatris, Panicum, Leptoloma cognatum, Quercus laevis, Quercus incana, Quercus geminata, Aristida stricta, Vaccinium stamineum, Vaccinium myrsinites and Licania michauxii. [6]
Phenology
Asclepias humistrata flowers March through October, with peak inflorescence in April.[7] Fruits April through October.[6]
Seed dispersal
The fruit dries and splits open, releasing seeds with a white, silky pappus that allows for wind dispersal.[1] The species is thought to be dispersed by wind.[8]
Seed bank and germination
Germination is delayed until late winter or spring when seeds have after-ripened and environmental temperatures increase to match seeds germination requirements.[9]
Fire ecology
It has been observed growing in burned over, longleaf pine forests.[6] A long, deep, thick taproot aids in rapid recovery after fire.[1]
Pollination
Pollination of Asclepias is unusual. Pollen is contained in sacs (pollinia) located in the slits of the flower (stigmatic slits), when a pollinator walks across the flower head, these sacs attach to the pollinator and disperses on to another plant when the pollinator lands and walks.[1] There is no specialist insect pollinator.[9]
Herbivory and toxicology
This species is an important host plant to the monarch butterfly (Danaus plexippus) and the Queen butterfly (Danaus gilippus). Monarch larva feed upon the milkweed plants, ingesting the plant defense chemicals (cardenolids). Monarch caterpillars have evolved the ability to circumvent latex defence of milkweeds and appropriate the cardenolids. However, they can only tolerate a certain level of the cardiac glycoside chemicals, larval survival is negatively correlated with the concentration of cardiac glycosides in the leaves. Many larvae will die because they can become adhered by the latex to the leaf surface.[10] Cohen and Brower (1982) observed that more eggs are laid on larger plants, however, larval success was greatest on smaller plants. There is a positive correlation between the number of eggs on a plant and total leaf area.[11]
Conservation, cultivation, and restoration
Cultural use
Photo Gallery
References and notes
- ↑ 1.0 1.1 1.2 1.3 1.4 [[2]]Florida Native Plant Society. Accessed: March 30, 2016
- ↑ 2.0 2.1 Weakley, A.S. 2015. Flora of the southern and mid-atlantic states. Working Draft of 21 May 2015. University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
- ↑ 3.0 3.1 Radford, Albert E., Harry E. Ahles, and C. Ritchie Bell. Manual of the Vascular Flora of the Carolinas. 1964, 1968. The University of North Carolina Press. 848-852. Print.
- ↑ 4.0 4.1 Diaz-Toribio, M.H. and F. E. Putz 2021. Underground carbohydrate stores and storage organs in fire-maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108: 432-442.
- ↑ Diaz-Torbio, M. H. and F. E. Putz. 2021. Underground carbohydrate stores and storage organs in fire-maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108(3):432-442.
- ↑ 6.0 6.1 6.2 6.3 Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: November 2015. Collectors: Loran C. Anderson, M. Boothe, Edwin L. Bridges, Richard Carter, Jack P. Davis, Elmer, J.P. Gillespie, Robert K. Godfrey, R.D. Houk, Lisa Keppner, Gary R. Knight, Robert Kral , H. Larry, Robert L. Lazor, Karen MacClendon, Sidney McDaniel, R.A. Norris, Steve L. Orzell, C. Prichard, Grady W. Reinert, Annie Schmidt, E. Stipling, D.B. Ward, S.J. Ward, Rodie White, Mary Margaret Williams, Jean W. Wooten. States and Counties: Florida: Baker, Bay, Calhoun, Clay, Dixie, Escambia, Franklin, Gadsden, Gilchrist, Hamilton, Jackson, Leon, Levy, Liberty, Marion, Nassau, Okaloosa, Pasco, Polk, Santa Rosa, Taylor, Volusia, Wakulla, Washington. Georgia: Grady. Compiled by Tall Timbers Research Station and Land Conservancy.
- ↑ Nelson, G. PanFlora: Plant data for the eastern United States with emphasis on the Southeastern Coastal Plains, Florida, and the Florida Panhandle. www.gilnelson.com/PanFlora/ Accessed: 19 MAY 2021
- ↑ Kirkman, L. Katherine. Unpublished database of seed dispersal mode of plants found in Coastal Plain longleaf pine-grasslands of the Jones Ecological Research Center, Georgia.
- ↑ 9.0 9.1 [[3]]Xerces Society. Accessed: March 30, 2016
- ↑ Zalucki, M. P., L. P. Brower, et al. (2001). "Detrimental effects of latex and cardiac glycosides on survival and growth of first-instar monarch butterfly larvae Danaus plexippus feeding on the sandhill milkweed Asclepias humistrata." ECOLOGICAL ENTOMOLOGY 26(2): 212-224.
- ↑ Cohen, J. A. and L. P. Brower (1982). "Oviposition and Larval Success of Wild Monarch Butterflies (Lepidoptera: Danaidae) in Relation to Host Plant Size and Cardenolide Concentration." Journal of the Kansas Entomological Society 55(2): 343-348.