Aristida stricta
| Aristida stricta | |
|---|---|

| |
| Photo by John R. Gwaltney, Southeastern Flora.com | |
| Scientific classification | |
| Kingdom: | Plantae |
| Division: | Tracheophyta - Vascular plants |
| Class: | Lilianae - Monoctyledons |
| Order: | Poales |
| Family: | Poaceae |
| Genus: | Aristida |
| Species: | A. stricta |
| Binomial name | |
| Aristida stricta L. | |
| |
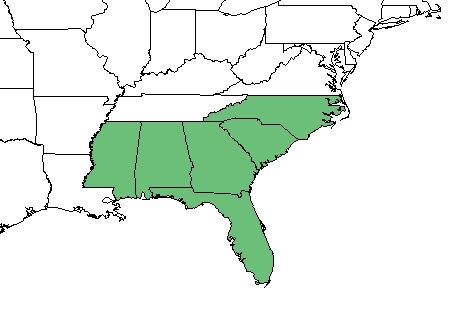
| Natural range of Aristida stricta from USDA NRCS Plants Database. | |
Common names: Threeawn, Wiregrass, Carolina wiregrass, pineland three-awn
Contents
Taxonomic notes
Synonyms: A. stricta var. stricta[1]
Varieties: A. beyrichiana Trinius & Ruprecht[1]
Description
A. stricta is a perennial bunch grass that ranges in size, up to 15 cm across at the base.[2] The blades are narrow, flat, involuted, and appear to be round like wire.[2] The leaves are rigid yet bendable and generally 0.5m long.[2] There are 2-3 leaves in each tiller.[3] The shallow, dense, wiry roots are adept at taking in nutrients.[2] It maintains an approximate density of five clumps per square meter.[2] The seeds are translucent brown, rough in texture, cylindrical like in shape but narrow at the ends, 4.5 mm in length and 0.4 mm in width.[2] Basal growth and flowering of A. stricta can be facilitated by surrounding groundcover and longleaf pines in sandhill and seepage slope habitats.[4] The closer A. stricta is to long leaf pines, the greater its potential reproductive output and biomass because of shade provided by long leafs which effects temperature, humidity, and water loss in the soil.[4][5][6][7][8]
Distribution
A. stricta is found in areas adjacent to the Coastal Plain, in the Piedmont areas in northeast North Carolina to northeast South Carolina and also in Florida, Georgia, southern Alabama, and southeast Mississippi.[9][10]
Ecology
Aristida stricta was historically a foundational grass species in the longleaf pine savannas of the eastern portion of the southeastern U.S. Coastal Plain[11] and remains in areas that have been frequently burned, have an open tree canopy, and lack a history of severe soil disturbance.[12] The foliage of A. stricta helps lightning-set fires to spread and thereby maintain habitats, pine savannas, sandhills, and pine flatwoods.[9] However, those habitats are not common due to agriculture, pine farms, and development.[9] Fire exclusion and disturbance has led to a rapid decline in A. stricta’s population throughout the Coastal Plain.[9] Weakley mentions Ward (2001) proposes there is varietal status for A. stricta and A. beyrichiana, see Weakley’s most recently updated guide. Wunderlin and Hansen (2011) mention that the Aristida stricta, in Florida, is var. beyrichiana.[13] A. stricta is important in longleaf ecosystem restoration because of the critical role it serves by increasing water holding capacity, improving soil structure, and providing a fuel source for fire.[4] [14] [15]
Habitat
Aristida stricta has been observed in dry and loamy sand in upland longleaf pine communities, including sandhills, flatwoods, glades, ridges, borders of swamps, scrub, and slightly disturbed areas.[16] It is considered to be an indicator species for native plant communities of pine-grasslands in the Coastal Plain within its natural geographic and edaphic distribution due to its high sensitivity to soil disturbance.[17]
A. stricta reduced its crown cover and biomass in response to heavy silvilculture and showed resistance to regrowth in reestablished native pine flatwoods in North Florida.[18] Additionally, it has reduced occurrence in habitats disturbed by agricultural practices. It has shown resistance to regrowth in reestablished longleaf pine communities that were disturbed by agriculture in southwest Georgia and North Carolina,[17] making the plant an indicator of soil degradation in post agricultural habitats.[19][20] A. stricta decreased its cover in response to soil disturbance by roller chopping in the Northwest Florida sandhills and south Florida.[21][22] However, in some areas of north Florida A. stricta had mixed responses to roller chopping disturbance. Some cases were dependent upon the time since soil disturbance as the plant showed some regrowth in reestablished native pine communities.[23] It is present in infertile sands such as inadequately drained flatwoods soils and exceptionally drained sandhill soils.[2][24]
Its biomass decreased in response to soil disturbance by chopping, disking, fertilization, and bedding in South Florida dry prairies, and it showed resistance to regrowth in reestablished prairies that were disturbed by these practices.[25] It also decreased its cover in response to soil disturbance by clearcutting and chopping in North Florida flatwoods forests. A. stricta was resistant to regrowth in the reestablished flatwood community post disturbance.[26] A. stricta decreased its cover in response to soil disturbance by double chopping with a double drum chopper, single chopping with a double drum chopper, and by application of hexazinone in the Central Florida sandhills. It also failed to reoccur in post disturbance areas.[27] Additionally, in North Florida A. stricta has been found to be a decreaser in its short-term response to single mechanical soil disturbances as well as in its long-term response following cessation of repeated soil disturbance.[28]
Additionally, A. stricta decreased its cover in response to burning, BSW blading, double chopping, chopping, double disking, rootraking with disking, and bedding disturbance in North Florida longleaf pine ecosystems. It also showed resistance to regrowth after the disturbed longleaf pine ecosystem was reestablished. However, the plant did not respond to burning with rootraking in the North Florida longleaf pine community.[24]
Associated species: Pinus palustris, Aristida rhizomophora, Sorghastrum sp., Panicum sp., Andropogon sp., Sporobolus floridanus, Ctenium sp., Sarracenia sp., Lachnanthes sp., Scleria sp., Rhynchospora sp., Eriogonum tomentosum, Eupatorium capillifolium, Licania michauxii, Quercus geminata, Quercus laevis, Serenoa repens, and Vaccinium arboreum.[16]
Phenology
A. stricta can flower throughout the year[29], although it typically flowers only following late spring-summer (April-August) fires, beginning in the late summer or autumn of the same year.[13] Flowering has also been observed to be induced by disturbances other than fire, including transplanting, partial damage to roots from ploughed firelines or track-laying vehicles, or defoliation (removal of leaves), but not defoliation alone.[3] The highest amount of inflorescence has been observed to occur in August and September following a May burn and after growing season fires.[3] [30]
Seed dispersal
Aristida stricta is thought to be dispersed by gravity.[31] While it was hypothesized that A. stricta seeds are dispersed by gopher tortoises (Gopherus polyphemus), a study in 2005 found that this seemed unlikely since the seeds mature on stalks about 1 m tall and mature between October and December when the tortoises are mostly inactive.[32]
Additionally, Aristida stricta can reproduce through cloning once it is established. The rhizomes of the tussocks will expand in a radial pattern and quickly become independent of the parent plant and each other. This method of reproduction is usually seen in desert plant species.[33]
Seed bank and germination
Seed viability falls out between 100–120˚C.[3] Typically seed viability can last up to four months.[34] Short-term seed persistence can occur in the soil.[35] Requires high temperature for germination (30–35˚C).[36] Successful germination occurs when seeds are buried less than 2 cm.[34] Following fire, seeds that are year old may germinate right away, whereas those four months old could take another hundred days to germinate.[3] Dry seed has a higher tolerance of heat than moist seed.[3] Summer burning triggers effective flowering.[2] The month of burn appears to affect the abundance of reproductive tiller (growth unit that gives rise to a stem, seedhead, roots, and leaves),[37] with May having the highest amount.[3] One study found that seedlings only occurred when the area was burned in the lightning season.[34] Germination rate and seedling count are highest in July and May.[38]
Fire ecology
A. stricta is very flammable because of its highly fibrous leaf structure, vast amount of leaves, and duration of dead leaves which do not detach quickly.[2] A. stricta can withstand fire suppression for 20 or 40 years.[2] However, the sexual reproduction of A. stricta relies on fire for its persistence.[39] A. stricta grows up to 2.5 cm per day following fire,[3] and populations have been known to persist through repeated annual burns.[40] Low intensity fires cause production of more culms (stems) per clump of A. stricta.[30] The spatial variation of fire intensity may have a high impact on A. stricta recruitment patterns.[30] With the correct fire regime a population of A. stricta can survive indefinitely, possibly germinating from seeds thousands of years ago,[2] although they can be killed by unusually severe fires.[41] One study observed when burns occurred during growing season and seedlings were closer to mature plants, mortality among seedlings was higher.[42] To establish successfully after germination, new seedlings have shown to need 1-2 years without growing season fires.[42] However, growing season burns within the first 2 years have been observed to result in high inflorescence and seed production.[42] Therefore for best rates of seedling survival, seeds can be produced with a growing season burn followed by 1-2 years without growing season fire to allow for seedling establishment.[42]
Herbivory and toxicology
A few weeks following fire, newly resprouted blades are palatable to cattle but afterwards become unappealing to grazers as they mature.[3][43] It consists of 2-5% of the diet of terrestrial birds, including Bachman's sparrows, in its community.[44][45] Deer are also known to browse the leaves.[44] The planthopper Delphacodes andromeda (family Delphacidae) has been observed to use this species as a host.[46]
Conservation, cultivation, and restoration
A. stricta has been observed to be negatively affected by soil disturbance.[47][48] A. stricta has also been observed to have a negative association with agricultural history and a positive association with burning frequency.[49] One study observed that A. stricta has lowest mortality when hexazinone (a herbicide) is used during dry times and when chopping is performed with high soil moisture when using these methods.[14] During one study A. stricta was subject to double chopping which drastically reduced its numbers and did not allow it recover even several years after the damage.[50] Aboveground damage to clones can be healed but serious root damage commonly leads to death.[14] The impact of site preparation on A. stricta can be reduced by using single-pass treatments, specifically with a single-drum chopper.[24] Single pass treatments can still diminish A. stricta in the long-term but double chopping, rootraking, and disking practices can eradicate A. stricta from an area for decades.[24] One study observed five years after 1-2 disking treatments, A. stricta returned to its original amount.[24] These soil disturbances cause exposure and extreme dryness of roots which damage and kill A. stricta.[24] As part of a longleaf pine and wiregrass community restoration project at the Apalachicola Bluffs and Ravines Preserve, The Nature Conservancy propagated A.stricta from seed and made many observations about the process.[51] Larger plants develop when propagating A. stricta plugs in a nursery when compared to direct seeding methods.[51] A growing season burn will provide seed to collect.[51] The best time for harvesting seed is before seed immaturity and after the stem has dropped viable seed.[51] After seed was collected, they were tested for viability on damp filter paper in petri dishes.[51] Germination started within 5-10 days for viable seed and testing occurred until seed was mature.[51] Seed was collected by hand from the flowering stalk.[51] Seeds were planted 1/4" deep in 3"x6" grow-tubes, covered with 30% shade cloth, and watered daily with a sprinkler hose.[51] The most successful plants developed from seed planted in soil containing 1 part native soil and 10 parts potting soil including a slow-release fertilizer.[51] Germination started in about 14 days.[51] 6-18 month A. stricta plugs were planted using a dibble stick or trowel.[51] 15 months after planting 9,000 plugs they had a survival rate of 99%.[51]
Andrews Nursery also propagated A. stricta seeds in a nursery but used a slightly different method. Seeds were removed from the stalk and put in petri dish with a cross-linked polymer gel.[52] Seeds germinated with natural light at room temperature within 12-30 days.[52] For faster germination a growth chamber at 30ºC can achieve germination within 6-12 days.[52] A prairie striper with a 0.065 head gauge was used to collect seed.[52] From November to mid December seeds were collected on low humidity days.[52] Seed was stored in barrels and used within 8 months to ensure viability.[52] Germination tests were performed in February or March with seeds and gel in plastic boxes. Seeds were planted after freezing weather but before hot weather in 98 ml growing containers with a mix of 5 parts peat moss, 3 parts polystyrene beads, 2 parts coarse vermiculite, and controlled release Osmocote.[52] A ribbon mixing machine was used to mix seed with vermiculite and then placed 6-12 mm deep in the growing containers.[52] The trays of growing containers were covered with a shade cloth and watered daily.[52] The shade cloth was removed 6-10 days later when seedlings reach a height of 25 mm.[52] Seedlings can be planted after 3 months and up to 12 months.[52]
A 37-year study conducted by the Tall Timbers Research Station found that wiregrass can be planted in an area in lower densities and still be able to eventually dominate the area on its own through reproduction. The growth trend was on a steady increase over the 37 years. Though that expansion was mostly contained within the originally planted area and did not laterally increase significantly, there was evidence supporting that given the necessary fire regime A stricta could potentially begin expanding laterally. The contained expansion of the study appeared to be due to most of the area gained being attributed to clonal reproduction instead of seed dispersal, and the plants regulate themselves to maintain between 4-6 individuals per 1m^2 plot. The overall implication of these results is that wiregrass species can be planted in low densities even for restoration projects, as they will self regulate to intermediate values of density, area, and size, and still will become the dominant cover over a 20 year period so long as management practices are appropriate for the species.[53]
Cultural use
Photo Gallery
References and notes
- ↑ 1.0 1.1 Weakley, A.S. 2020. Flora of the Southeastern United States. Edition of 20 October 2020. University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 Clewell, A. F. (1989). Natural History of Wiregrass (Aristida stricta Michx., Gramineae). Natural Areas Journal 9: 223-233.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 Parrott, R. T. (1967). A study of wiregrass (Aristida stricta Mitchx.) with particular reference to fire, MA Thesis, Duke University: 137.
- ↑ 4.0 4.1 4.2 Wallett, W. D. (2015). Neighborhood interactions of an understory dominant, Aristida stricta, along a soil resource gradient of the long leaf pine ecosystem. Biological Sciences Murray, Murray State University Master of Science 74.
- ↑ Callaway, R.M. (2007) Positive Interactions and Interdependence in Plant Communities. Springer, Dordrecht.
- ↑ Callaway, R.M., & Walker, L.R. (1997) Competition and facilitation: a synthetic approach to Interactions in plant communities. Ecology, 78, 1958–1965.
- ↑ Espeleta, J., West, J., & Donovan, L. (2004). Species-specific patterns of hydraulic lift in co-occurring adult trees and grasses in a sandhill community. Oecologia, 138, 341-349.
- ↑ McGuire, J.P., Mitchell, R.J., Moser, E.B., Pecot, S.D., Gjerstad, D.H., & Hedman, C.W. (2001). Gaps in a gappy forest: plant resources, longleaf pine regeneration, and understory response to tree removal in longleaf pine savannas. Can. J. For. Res. 31, 765–778.
- ↑ 9.0 9.1 9.2 9.3 Weakley, Alan S. Flora of the Southern and Mid-Atlantic States: Working Draft of 21 May 2015. University of North Carolina Herbarium (NCU). PDF. 358; 360.
- ↑ USDA, NRCS. 2016. The PLANTS Database (http://plants.usda.gov, 5 December 2016). National Plant Data Team, Greensboro, NC 27401-4901 USA.
- ↑ Fill, J. M., B. M. Moule, M. J. Varner and T. A. Mousseau 2016. Flammability of the keystone savanna bunchgrass Aristida stricta. Plant Ecology 217: 331-342.
- ↑ Clewell, A. F. 1989. Natural history of wiregrass (Aristida stricta Michx., Gramineae). Natural Areas Journal 9: 223-233.
- ↑ 13.0 13.1 Wunderlin, Richard P. and Bruce F. Hansen. Guide to the Vascular Plants of Florida. Third edition. 2011. University Press of Florida: Gainesville/Tallahassee/Tampa/Boca Raton/Pensacola/Orlando/Miami/Jacksonville/Ft. Myers. 178. Print.
- ↑ 14.0 14.1 14.2 Outcalt, K. W. (1992). "Factors affecting wiregrass (Aristida stricta Michx.) cover on uncut and site prepared sandhills areas in Central Florida. Ecological Engineering 1: 245-251.
- ↑ Clewell, A. F. (1989). "Natural History of Wiregrass (Aristida stricta Michx., Gramineae)." Natural Areas Journal 9: 223-233.
- ↑ 16.0 16.1 Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: March 2019. Collectors: Jame Amoroso, Loran C. Anderson, Wilson Baker, L. Baltzell, S. T. Cooper, A. H. Curtiss, R. A. Davidson, Patricia Elliot, J. P. Gillespie, R. K. Godfrey, Frank W. Gould, J. Hunter, Gary R. Knight, R. Komarek, R. Kral, Robert L. Lazor, Karen MacClendon, D. L. Martin, R. S. Mitchell, John Morrill, John B. Nelson, R. E. Perdue, Jr., D. Pheilps, Gwynn W. Ramsey, William Reese, Paul Redfearn, Cecil R. Slaughter, Brian Tan, R. F. Thorne, D. B. Ward, F. S. Ward, and G. Wilder. States and Counties: Florida: Bay, Brevard, Calhoun, Columbia, Duval, Escambia, Franklin, Gadsden, Gulf, Indian River, Jackson, Jefferson, Lake, Leon, Levy, Liberty, Madison, Marion, Nassau, Osceola, Pasco, Pinellas, Polk, Putnam, Suwannee, Taylor, Wakulla, Walton, and Washington. Georgia: Thomas.
- ↑ 17.0 17.1 Ostertag, T.E., and K.M. Robertson. 2007. A comparison of native versus old-field vegetation in upland pinelands managed with frequent fire, South Georgia, USA. Pages 109–120 in R.E. Masters and K.E.M. Galley (eds.). Proceedings of the 23rd Tall Timbers Fire Ecology Conference: Fire in Grassland and Shrubland Ecosystems.
- ↑ Conde, L.F., B.F. Swindel, and J.E. Smith. (1986). Five Years of Vegetation Changes Following Conversion of Pine Flatwoods to Pinus elliottii Plantations. Forest Ecology and Management 15(4):295-300.
- ↑ Hedman, C.W., S.L. Grace, and S.E. King. (2000). Vegetation composition and structure of southern coastal plain pine forests: an ecological comparison. Forest Ecology and Management 134:233-247.
- ↑ Brudvig, L. A., J. L. Orrock, Damschen, C. D. Collins, P. G. Hahn, W. B. Mattingly, J. W. Veldman and J. L. Walker. 2014. Land-use history and contemporary management inform an ecological reference model for longleaf pine woodland undestory plant communities. PLoS ONE 9(1): e86604.
- ↑ Hebb, E.A. (1971). Site Preparation Decreases Game Food Plants in Florida Sandhills. The Journal of Wildlife Management 35(1):155-162.
- ↑ Lewis, C.E. (1970). Responses to Chopping and Rock Phosphate on South Florida Ranges. Journal of Range Management 23(4):276-282.
- ↑ Lewis, C.E., G.W. Tanner, and W.S. Terry. (1988). Plant responses to pine management and deferred-rotation grazing in north Florida. Journal of Range Management 41(6):460-465.
- ↑ 24.0 24.1 24.2 24.3 24.4 24.5 Outcalt, K.W. and C.E. Lewis. 1990. Response of wiregrass (Aristida stricta) to mechanical site preparation. In L.C. Duever and R.F. Noss (Editors). Proceedings of a symposium on wiregrass biology and management, October 13, 1988, Valdosta, Georgia. KBN Engineering and Applied Sciences, Gainesville, Florida. 12 pages.
- ↑ Moore, W.H. and B.F. Swindel. (1981). Effects of Site Preparation on Dry Prairie Vegetation in South Florida. Southern Journal of Applied Forestry 27(2)89-92.
- ↑ Moore, W.H., B.F. Swindel, and W.S. Terry. (1982). Vegetative Response to Clearcutting and Chopping in a North Florida Flatwoods Forest. Journal of Range Management 35(2):214-218.
- ↑ Outcalt, K.W. (1992). Factors affecting wiregrass (Aristida stricta Michx.) cover on uncut and site prepared sandhills areas in Central Florida. Ecological Engineering 1(3):245-251.
- ↑ Dixon, C. M., K. M. Robertson, A. M. Reid and M. T. Rother. 2024. Mechanical soil disturbance in a pine savanna has multiyear effects on plant species composition. Ecosphere 15(2):e4759.
- ↑ Nelson, G. Panflora: Plant database for the eastern United States with an emphasis on the Southeastern Coastal Plains, Florida, and the Florida pandhandle. Accessed 5 DEC 2016.
- ↑ 30.0 30.1 30.2 Jeff S. Glitzenstein, D. R. S., William J. Platt (1995). Evaluating the effects of season burn on vegetation in longleaf pine savannas Tallahassee, Florida Game and Fresh Water Fish Comission.
- ↑ Kirkman, L. Katherine. Unpublished database of seed dispersal mode of plants found in Coastal Plain longleaf pine-grasslands of the Jones Ecological Research Center, Georgia.
- ↑ Birkhead, R. D., et al. (2005). "Patterns of folivory and seed ingestion by gopher tortoises (Gopherus polyphemus) in a southeastern pine savanna." American Midland Naturalist 154: 143-151.
- ↑ Laucevivius Jr., A.M.; Robertson, K.M.; Means, D.B.; Mitchell, T.R.; Taylor, P.B. 2021. Expansion and population structure of transplanted Aristida beyrichiana (wiregrass) tussocks: results of a 37-year study.
- ↑ 34.0 34.1 34.2 McGee, A. J. (1997). Seed ecology of bunchgrasses of longleaf pine - wiregrass communities at Fort Stewart, Georgia. Biology. Statesboro, Georgia Southern University. Master of Science: 85.
- ↑ Coffey, K. L. and L. K. Kirkman (2006). "Seed germination strategies of species with restoration potential in a fire-maintained pine savanna." Natural Areas Journal 26: 289-299.
- ↑ Andreu, M. G., C. W. Hedman, et al. 2009. "Can managers bank on seed banks when restoring Pinus taeda L. plantations in Southwest Georgia?" Restoration Ecology. Vol 17. pgs 586-596.
- ↑ Trlica, M. J. (1999). "Grass growth and response to grazing ". from http://extension.colostate.edu/topic-areas/natural-resources/grass-growth-and-response-to-grazing-6-108/.
- ↑ Eerden, B. P. V. (1997). Studies on the reproductive biology of wiregrass (Aristida stricta Michaux) in the Carolina sandhills. Athens, University of Georgia. Master of Science: 89.
- ↑ Jennifer M. Fill, et al. (2012). The reproductive response of an endemic bunchgrass indicates historical timing of a keystone process. Ecosphere 3: 1-12.
- ↑ Robertson, K.M. Unpublished data collected from Pebble Hill Fire Plots, Pebble Hill Plantation, Thomasville, Georgia.
- ↑ Robertson, Kevin M. 2015. Unpublished data from the Pebble Hill Fire Plots (Pebble Hill Plantation, near Thomasville, Georgia) indicating death of some genetic individuals of A. stricta after a prescribed burn in June after five years without fire.
- ↑ 42.0 42.1 42.2 42.3 Mulligan, M. K. and L. K. Kirkman (2002). "Burning influences on wiregrass (Aristida beyrichiana) restoration plantings: natural seedling recruitment and survival." Restoration Ecology 10(2): 334-339.
- ↑ Lewis, C. E. (1970). Responses to chopping and rock phosphate on south Florida ranges. Journal of Range Management 23: 276-282.
- ↑ 44.0 44.1 [[1]] Lady Bird Johnson Wildflower Center. Accessed: March 15, 2019
- ↑ Miller, J.H., and K.V. Miller. 1999. Forest plants of the southeast and their wildlife uses. Southern Weed Science Society.
- ↑ Discoverlife.org [2]
- ↑ Hebb, E. A. (1971). Site preparation decreases game food plants in Florida sandhills. Journal of Wildlife Management 35: 155-162.
- ↑ Kirkman, L. K., K. L. Coffey, et al. (2004). "Ground cover recovery patterns and life-history traits: implications for restoration obstacles and opportunities in a species-rich savanna." Journal of Ecology 92(3): 409-421.
- ↑ C. W. Hedman, S. L. G., and S.E. King (2000). Vegetation composition and structure of southern coastal plain pine forests: an ecological comparison. Forest Ecology and Management 134: 233-247.
- ↑ Hebb, E. A. (1971). "Site preparation decreases game food plants in Florida sandhills." Journal of Wildlife Management 35: 155-162.
- ↑ 51.00 51.01 51.02 51.03 51.04 51.05 51.06 51.07 51.08 51.09 51.10 51.11 Seamon, P. A. and R. L. Myres (1992). "Propogating wiregrass from seed." The Palmetto(Winter 1992): 6-7.
- ↑ 52.00 52.01 52.02 52.03 52.04 52.05 52.06 52.07 52.08 52.09 52.10 Pittman, T. and R. P. Karrfalt (1998). "Wiregrass propagation at the Andrews Nursery in Florida." 1-3.
- ↑ Laucevivius Jr., A.M.; Robertson, K.M.; Means, D.B.; Mitchell, T.R.; Taylor, P.B. 2021. Expansion and population structure of transplanted Aristida beyrichiana (wiregrass) tussocks: results of a 37-year study.