Centrosema virginianum
| Centrosema virginianum | |
|---|---|

| |
| Photo by Gil Nelson | |
| Scientific classification | |
| Kingdom: | Plantae |
| Division: | Magnoliophyta - Flowering plants |
| Class: | Magnoliopsida – Dicotyledons |
| Order: | Fabales |
| Family: | Fabaceae ⁄ Leguminosae |
| Genus: | Centrosema |
| Species: | C. virginianum |
| Binomial name | |
| Centrosema virginianum (L.) Benth. | |
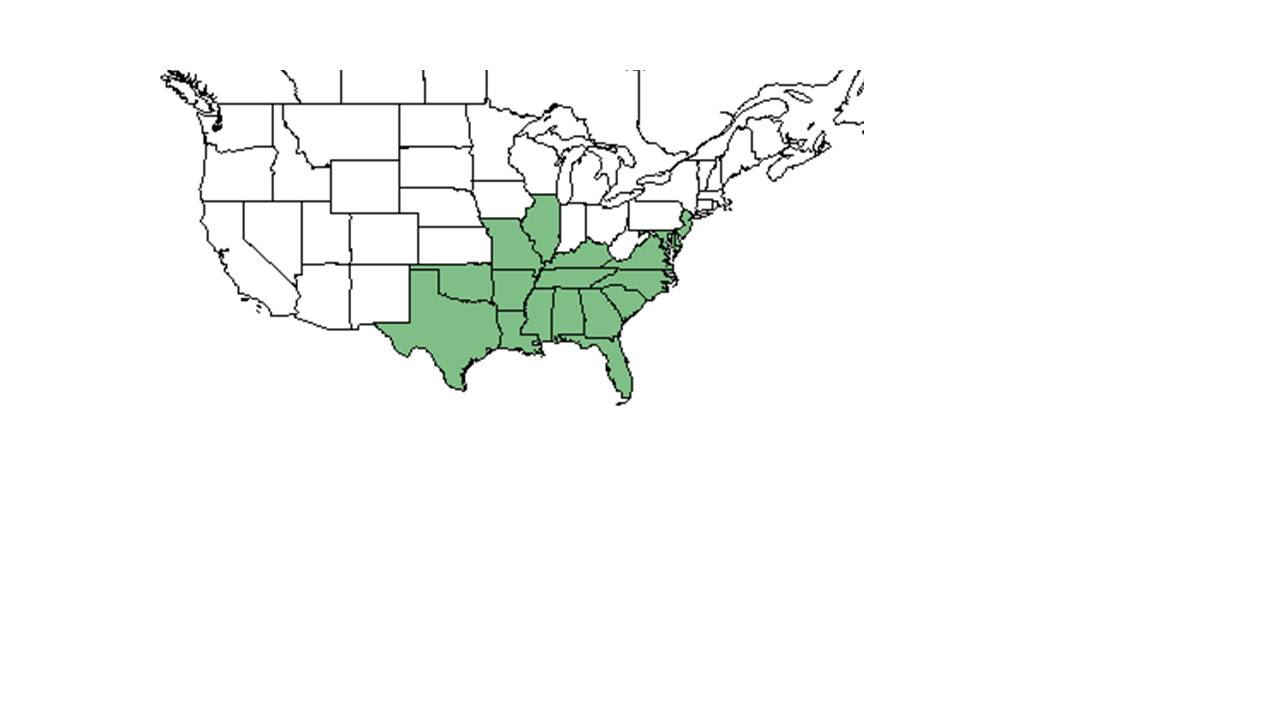
| |
| Natural range of Centrosema virginianum from USDA NRCS Plants Database. | |
Common name: Spurred butterfly pea
Contents
Taxonomic notes
Synonyms: Bradburya virginiana (Linnaeus) Kuntze[1]
Varieties: Centrosema virginianum;; var. angustifolium (A.P. de Candolle) Grisebach; C. virginianum var. ellipticum Fernald; C. virginianum var. virginianum[1]
Description
Centrosema virginianum is a perennial herbaceous vine seen growing in a tailing, climbing, and twining fashion between 0.5 - 1.5 m long and is more or less minutely pubescent throughout. The leaves are 3-foliolate with leaflets widely to narrowly ovate, ovate-lanceolate or oblong to elliptic; they are conspicuously reticulate, mostly growing 2 - 7 cm long, and are stipellate. The stipules are ovate-lanceolate to lanceolate, striate, persistent, 1.5 - 4 mm long. The racemes are with peduncles usually growing 1 - 5 cm long, the zig-zag flower stalk bears 1 - 4 nodes each with an ovate bract growing up to 1.5 - 3 cm long supporting a pedicel (growing 2 - 10 cm long) surmounted by 2 ovate bractlets growing 0.8 - 1.2 cm long. The calyx is somewhat hidden by the bractlets and the tube is broadly hemispheric, growing 4 - 5 mm long, with 0.6 - 1.4 cm long lobes that are linear-subulate with the lowermost being longest. The petals are pale blue-violet to lavender in color, growing 2.5 - 3.5 cm long, is spurred near the base, and the wigs and keel are nearly equal in size (ca. 2 cm long). The stamens are diadelphous, 9 and 1. The legume is linear, flattened, and grows 7 - 14 cm long and ca. 4 mm broad, has a small stalk, and contains many seed with an elongate, persistent, beak-like style, valves longitudinally twisting after dehiscence.[2][3] The leaflet langth to width ratio is very variable.[4]
The root system of Centrosema virginianum includes stem tubers which store non-structural carbohydrates (NSC) important for both resprouting following fire and persisting during long periods of fire exclusion.[5] Diaz-Toribio and Putz (2021) recorded this species to have an NSC concentration of 399 mg/g (ranking 6 out of 100 species studied) and water content of 57.4% (ranking 31 out of 100 species studied).[5]
Distribution
C. virginianum is native to the southeast United States, north up to Illinois and New Jersey, along the east coast and south to Florida, and west to Texas and Oklahoma. It is also native to Puerto Rico as well as the Virgin Islands.[6]
Ecology
Centrosema virginianum is a legume with one of the highest nitrogen-fixing potentials.[7] Because of this, it may be able to help restore N lost from fire.[8] By mid-season in June and July, a maximum nitrogen-fixing rate was observed.[7]
Habitat
C. virginianum is found in a wide range of natural and disturbed conditions, including frequently burned sandhills, upland longleaf-wiregrass and old-field pinelands[8][9] and flatwoods, coastal island dunes and shorelines, open areas within mangrove swamps, wooded floodplains and edges of hardwood forests, bogs, and loblolly pine communities.[9][8] C. virginianum is a characteristic species of the shortleaf pine-oak-hickory community.[10]
This species is tolerant of overstory canopies that decrease the light level to about half the ambient (i.e., it can live in partially shaded areas and its nitrogen-fixing capability won't be significantly affected).[7] It can grow highly disturbed areas, but it is ubiquitous in high quality native longleaf pine uplands and sandhills, occurring in soil types ranging from deep sands (Entisols) to sandy loams (Ultisols). A study exploring longleaf pine patch dynamics found C. virginianum to be most strongly represented within stands of longleaf pine that are between 90-250 years of age.[11]
C. virginianum increased its occurrence in response to soil disturbance by agriculture in the coastal plains of South Carolina. It has also shown regrowth in reestablished longleaf pine woodlands that were disturbed by agricultural practices, making it an indicator species of post-agricultural woodland.[12][13] It does not respond to soil disturbance by clearcutting and chopping in North Florida flatwoods forests.[14]
Associated species includes Blackberry Bramble, turkey oak, longleaf pine, Galactia, Strophostyles, Smilax, Penstemon, Lechea, Chrysopsis, Brumelia, Centrosema, Euphorbia, Cassia, Serenoa repens, Quercus incana, Quercus chapmanii, Diospyros, Aristida, Andropogon, bahia grass, Rubus, cloverleaf, Pinus taeda, Liquidambar styraciflua, and others.[3]
Phenology
C. virginianum has been observed flowering between April and October, with peak inflorescence in June and July, and fruits primarily in June thorugh September.[3][15]
Seed dispersal
This species is thought to be dispersed by ants and/or explosive dehiscence. [16]
Seed bank and germination
It spreads clonally by production of rhizomes.[17] Seed coats are hard and and seeds and remain viable in the seed bank for at least two years.[18] For optimal germination, C. virginianum needs about 2 seconds of scarification time. It was also found to have greatest dry heat germination at a heat index between 128.76 and 191.83, and steam duration of 10 seconds.[19] With a study on the effects of variation in fine fuel loads on post-burn germination, it was found to have greater germination in low fuel than high fuel with a lower mortality rate as well, although the greatest rate of germination and lowest mortality was with the control of no burn regiment.[20]
Fire ecology
C. virginianum has been found to thrive under fire.[9] Hendricks observed that the Piedmont National Wildlife Refuge plots, which had been under a 4-year burning regime since 1966, each contained more than 10 times more C. virginianum individuals per hectare than the Oconee National Forest plots, which had no burning history.[21] Additionally, populations of Centrosema virginianum have been known to persist through repeated annual burns.[22][23]
Seasonal burning does not seem to negatively affect nitrogen fixation[24]; a study describing the effects of a seasonal fire regime on legume reproduction in longleaf pine savannas found that C. virginianum produces the greatest number of flowers after instances without fire (6.8) and decreases after a late winter/early spring burn (5.7) and after a lightning-season burn (3.9).[25] The study also found that the duration of synchronous flowering was greatest after instances without fire (73.0 days) and decreased after a late winter/early spring burn (67.3 days) and after a lightning-season burn (42.7 days).[26]
Additionally, the peak flowering activity occurred earliest after instances without fire (216.7 Julian) and occurred later after a late winter/early spring burn (231.0 Julian) and after a lightning-season burn (246.0 Julian).[27] C. virginianum has a mid-summer flowering peak[24] and was found to respond the best to March burns with respect to annual tissue inputs as well as nitrogen contribution.[24] One study found no evidence that increased flowering affects nitrogen-fixing capability.[24]
Pollinations
The flower of Centrosema virginianum is highly specialized for pollination by large Hymenoptera.[28] It requires bees for pollination to "trip" the pollen delivery mechanism. Pollinator-plant relationships appear to be robust to alteration in flowering phenology resulting from variation in season of burn.[24]Bombus pennsylvanicus was observed feeding and collecting pollen.[29]
Herbivory and toxicology
Because C. virginianum is a legume, and legumes are high in protein and mineral content, a number of herbivores including but not limited to Gopherus polyphemus, white-tailed deer, and bob-white quail, consume it.[8] One study found that it is a significantly important plant in 1-year and 2-years stands for the bobwhite quail diet.[30] It averages to be about 10-25% of the diet for large mammals and terrestrial birds.[31]
Diseases and parasites
C. virginianum can be infected by the root-knot nematode species Meloidogyne arenaria, M. incognita, and M. javanica, but it is moderately resistant.[32]
Conservation, cultivation, and restoration
It is listed as endangered by the New Jersey Department of Environmental Protection and Energy.[6] C. virginianum is tolerant to the herbicide imazapyr.[33] Also for management, it benefits from the overstory canopy being thinned.[34]
Cultural use
Photo Gallery
References and notes
- ↑ 1.0 1.1 Weakley, A.S. 2020. Flora of the Southeastern United States. Edition of 20 October 2020. University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
- ↑ Radford, Albert E., Harry E. Ahles, and C. Ritchie Bell. Manual of the Vascular Flora of the Carolinas. 1964, 1968. The University of North Carolina Press. 635-6. Print
- ↑ 3.0 3.1 3.2 Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: June 2014. Collectors: Loran C. Anderson, John C. Ogden, Gwynn W. Ramsey, R.K. Godfrey, R. S. Mitchell; R. C. Phillips, K. Craddock Burks, Gary R. Knight, D. W. Mather, C. Jackson, D. B. Ward, Mary Margaret Williams, O. Lakela, Brenda Herring, Jame Amoroso, Gwynn W. Ramsey, Richard Mitchell, Gail A. Steverson, Grady W. Reinert, George R. Cooley, R. J. Eaton, R. Kral, Cecil R Slaughter, Andre F. Clewell, R. Komarek, R. F. Doren, Kevin Oakes, Richard Gaskalla, Lisa Keppner, Clarke Hudson, Wilbur H Duncan, Jean Wooten, H. R. Totten, R. L. Wilbur, C. Ritchie Bell, Delzie Demaree, F. S. Earle, A. B. Seymour, Samuel B. Jones, Jr., H. R. Reed, A. B. Seymour, Michael B. Brooks, Sidney McDaniel, D. C. Bain, D. S. Correll, H. B. Correll, Lloyd H. Shinners, Geo M. Merrill, and H J Hamby. States and Counties: Alabama: Baldwin. Arkansas: Little Rock. Florida: Bay, Citrus, Collier, Duval, Escambia, Franklin, Gadsden, Gulf, Hillsborough, Jackson, Jefferson, Leon, Liberty, Manatee, Marion, Okaloosa, Polk, St Johns, St. Lucie, Suwannee, Wakulla, and Washington. Georgia: Bartow, Grady, Madison, and Thomas. Mississippi: Forrest, Harrison, Jackson, Pearl River, and Pike. North Carolina: Alamance, Orange, and Wilkes. Texas: Angelina, Bastrop, Freestone, Harris, Morris, Tarrant, and Van Zandt.
- ↑ Weakley, A. S. (2015). Flora of the Southern and Mid-Atlantic States. Chapel Hill, NC, University of North Carolina Herbarium.
- ↑ 5.0 5.1 Diaz-Toribio, M.H. and F. E. Putz 2021. Underground carbohydrate stores and storage organs in fire-maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108: 432-442.
- ↑ 6.0 6.1 USDA, NRCS. (2016). The PLANTS Database (http://plants.usda.gov, 4 April 2019). National Plant Data Team, Greensboro, NC 27401-4901 USA.
- ↑ 7.0 7.1 7.2 Cathey, S. E., L. R. Boring, et al. (2010). "Assessment of N2 fixation capability of native legumes from the longleaf pine-wiregrass ecosystem." Environmental and Experimental Botany 67: 444-450.
- ↑ 8.0 8.1 8.2 8.3 Hainds, M. J., R. J. Mitchell, et al. (1999). "Distribution of native legumes (Leguminoseae) in frequently burned longleaf pine (Pinaceae)-wiregrass (Poaceae) ecosystems." American Journal of Botany 86: 1606-1614.
- ↑ 9.0 9.1 9.2 Cushwa, C. T. (1966). The response of herbaceous vegetation to prescribed burning. Asheville, USDA Forest Service.
- ↑ Clewell, A. F. (2013). "Prior prevalence of shortleaf pine-oak-hickory woodlands in the Tallahassee red hills." Castanea 78(4): 266-276.
- ↑ Mugnani et al. (2019). “Longleaf Pine Patch Dynamics Influence Ground-Layer Vegetation in Old-Growth Pine Savanna”.
- ↑ Brudvig, L.A. and E.I. Damchen. (2011). Land-use history, historical connectivity, and land management interact to determine longleaf pine woodland understory richness and composition. Ecography 34: 257-266.
- ↑ Brudvig, L.A., E Grman, C.W. Habeck, and J.A. Ledvina. (2013). Strong legacy of agricultural land use on soils and understory plant communities in longleaf pine woodlands. Forest Ecology and Management 310: 944-955.
- ↑ Moore, W.H., B.F. Swindel, and W.S. Terry. (1982). Vegetative Response to Clearcutting and Chopping in a North Florida Flatwoods Forest. Journal of Range Management 35(2):214-218.
- ↑ Nelson, G. PanFlora: Plant data for the eastern United States with emphasis on the Southeastern Coastal Plains, Florida, and the Florida Panhandle. www.gilnelson.com/PanFlora/ Accessed: 7 DEC 2016
- ↑ Kirkman, L. Katherine. Unpublished database of seed dispersal mode of plants found in Coastal Plain longleaf pine-grasslands of the Jones Ecological Research Center, Georgia.
- ↑ Hiers, J. K. and R. J. Mitchell (2007). "The influence of burning and light availability on N-2-fixation of native legumes in longleaf pine woodlands." Journal of the Torrey Botanical Society 134: 398-409.
- ↑ Coffey, K. L. and L. K. Kirkman (2006). "Seed germination strategies of species with restoration potential in a fire-maintained pine savanna." Natural Areas Journal 26: 289-299.
- ↑ Wiggers, M. S., et al. (2017). "Seed heat tolerance and germination of six legume species native to a fire-prone longleaf pine forest." Plant Ecology 218: 151-171.
- ↑ Wiggers, M. S., et al. (2013). "Fine-scale variation in surface fire environment and legume germination in the longleaf pine ecosystem." Forest Ecology and Management 310: 54-63.
- ↑ Hendricks, J. J. and L. R. Boring (1999). "N2-fixation by native herbaceous legumes in burned pine ecosystems of the southeastern United States." Forest Ecology and Management 113: 167-177.
- ↑ Robertson, K.M. Unpublished data collected from Pebble Hill Fire Plots, Pebble Hill Plantation, Thomasville, Georgia.
- ↑ Glitzenstein, J. S., D. R. Streng, R. E. Masters, K. M. Robertson and S. M. Hermann 2012. Fire-frequency effects on vegetation in north Florida pinelands: Another look at the long-term Stoddard Fire Research Plots at Tall Timbers Research Station. Forest Ecology and Management 264: 197-209.
- ↑ 24.0 24.1 24.2 24.3 24.4 Hiers, J. K., R. J. Mitchell, et al. (2003). "Legumes native to longleaf pine savannas exhibit capacity for high N2-fixation rates and negligible impacts due to timing of fire." New Phytologist 157: 327-338
- ↑ Hiers, J. K., et al. (2000). "The effects of fire regime on legume reproduction in longleaf pine savannas: is a season selective?" Oecologia 125: 521-530.
- ↑ Hiers, J. K., et al. (2000). "The effects of fire regime on legume reproduction in longleaf pine savannas: is a season selective?" Oecologia 125: 521-530.
- ↑ Hiers, J. K., et al. (2000). "The effects of fire regime on legume reproduction in longleaf pine savannas: is a season selective?" Oecologia 125: 521-530.
- ↑ Spears, Jr. E. E. 1987. Island and mainland pollination ecology of Centrosema virginianum and Opuntia stricta. J. Ecol. 75: 351-362.
- ↑ Godts J.E. 1990 The Upside-Down Flower Palmetto 10(4):3
- ↑ Sweeney, J. M., et al. (1981). Bobwhite quail food in young Arkansas loblolly pine plantations. Arkansas Experiment Station bulletin 852. Fayetteville, AR, University of Arkansas, Divisionn of Agriculture, Agricultural Experiment Station.
- ↑ Miller, J.H., and K.V. Miller. 1999. Forest plants of the southeast and their wildlife uses. Southern Weed Science Society.
- ↑ Quesenberry, K. H., et al. (2008). "Response of native southeastern U.S. legumes to root-knot nematodes." Crop Science 48: 2274-2278.
- ↑ (2000). The role of fire in nongame wildlife management and community restoration: Traditional uses and new directions, Nashville, TN, USDA Forest Service, Northeastern Research Station.
- ↑ Brockway, D. G. and C. E. Lewis (2003). "Influence of deer, cattle grazing and timber harvest on plant species diversity in a longleaf pine bluestem ecosystem." Forest Ecology and Management 175: 49-69.