Difference between revisions of "Clitoria mariana"
HaleighJoM (talk | contribs) (→Ecology) |
Adam.Vansant (talk | contribs) (→Habitat) |
||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 18: | Line 18: | ||
}} | }} | ||
| − | Common names: Atlantic | + | Common names: Atlantic pigeonwings, butterfly pea, she-pea, Florida butterfly pea, Florida she-pea |
==Taxonomic notes== | ==Taxonomic notes== | ||
| − | Synonym: | + | Synonym: none<ref name=weakley>Weakley, A.S. 2020. Flora of the Southeastern United States. Edition of 20 October 2020. University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.</ref> |
| − | Varieties: ''Clitoria mariana'' Linnaeus ''var. mariana''; ''Clitoria mariana'' Linnaeus ''var. pubescentia'' | + | Varieties: ''Clitoria mariana'' Linnaeus ''var. mariana''; ''Clitoria mariana'' Linnaeus ''var. pubescentia''<ref name=weakley/> |
==Description== | ==Description== | ||
| Line 33: | Line 33: | ||
The root system of ''Clitoria mariana'' includes stem tubers which store non-structural carbohydrates (NSC) important for both resprouting following fire and persisting during long periods of fire exclusion.<ref name="Diaz"> Diaz-Toribio, M.H. and F. E. Putz 2021. Underground carbohydrate stores and storage organs in fire-maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108: 432-442.</ref> Diaz-Toribio and Putz (2021) recorded this species to have an NSC concentration of 412.9 mg/g (ranking 4 out of 100 species studied) and water content of 69.5% (ranking 18 out of 100 species studied).<ref name = "Diaz"/> | The root system of ''Clitoria mariana'' includes stem tubers which store non-structural carbohydrates (NSC) important for both resprouting following fire and persisting during long periods of fire exclusion.<ref name="Diaz"> Diaz-Toribio, M.H. and F. E. Putz 2021. Underground carbohydrate stores and storage organs in fire-maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108: 432-442.</ref> Diaz-Toribio and Putz (2021) recorded this species to have an NSC concentration of 412.9 mg/g (ranking 4 out of 100 species studied) and water content of 69.5% (ranking 18 out of 100 species studied).<ref name = "Diaz"/> | ||
| + | |||
| + | According to Diaz-Torbio and Putz (2021), ''Clitoria mariana'' has stem tubers with a below-ground to above-ground biomass ratio of 0.74 and nonstructural carbohydrate concentration of 412.9 mg g<sup>-1</sup>.<ref>Diaz‐Toribio, M. H. and F. E. Putz. 2021. Underground carbohydrate stores and storage organs in fire‐maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108(3):432-442.</ref> | ||
==Distribution== | ==Distribution== | ||
| Line 41: | Line 43: | ||
''C. mariana'' is listed as a facultative upland species, and mostly occurs in non-wetland areas such as frequently burned longleaf pine-turkey oak sandhills,<ref name="fsu"/> sand pine scrub (Entisols), <ref name=Greenberg> Greenberg, C. H. (2003). "Vegetation recovery and stand structure following a prescribed stand-replacement burn in sand pine scrub." Natural Areas Journal 23: 141-151. </ref> flatwoods (Spodosols) <ref name=bc03> Brewer, J. S. and S. P. Cralle (2003). "Phosphorus addition reduces invasion of a longleaf pine savanna (southeastern USA) by a non-indigenous grass (''Imperata cylindrica'')." Plant Ecology 167: 237-245. </ref> upland longleaf pine-wiregrass communities (Ultisols), and in the margins of mixed hardwood communities<ref name="fsu"/> and loblolly pine plantations. <ref name=cushwa> Cushwa, C. T. (1966). The response of herbaceous vegetation to prescribed burning. Asheville, USDA Forest Service. </ref>, but this species can occasionally occur in wetlands.<ref name= "USDA"/> | ''C. mariana'' is listed as a facultative upland species, and mostly occurs in non-wetland areas such as frequently burned longleaf pine-turkey oak sandhills,<ref name="fsu"/> sand pine scrub (Entisols), <ref name=Greenberg> Greenberg, C. H. (2003). "Vegetation recovery and stand structure following a prescribed stand-replacement burn in sand pine scrub." Natural Areas Journal 23: 141-151. </ref> flatwoods (Spodosols) <ref name=bc03> Brewer, J. S. and S. P. Cralle (2003). "Phosphorus addition reduces invasion of a longleaf pine savanna (southeastern USA) by a non-indigenous grass (''Imperata cylindrica'')." Plant Ecology 167: 237-245. </ref> upland longleaf pine-wiregrass communities (Ultisols), and in the margins of mixed hardwood communities<ref name="fsu"/> and loblolly pine plantations. <ref name=cushwa> Cushwa, C. T. (1966). The response of herbaceous vegetation to prescribed burning. Asheville, USDA Forest Service. </ref>, but this species can occasionally occur in wetlands.<ref name= "USDA"/> | ||
| − | ''C. mariana'' ranges from dry<ref name=wp> Walker, J. and R. K. Peet (1983). "Composition and species diversity of pine-wiregrass savannas of the Green Swamp, North Carolina." Vegetation 55: 163-179. </ref> to moist sand soils,<ref name="fsu"/> and thought it typically lives in high light areas, it can also live in partially shaded areas (54% ambient light conditions).<ref name=Cathey> Cathey, S. E., L. R. Boring, et al. (2010). "Assessment of N2 fixation capability of native legumes from the longleaf pine-wiregrass ecosystem." Environmental and Experimental Botany 67: 444-450. </ref><ref name="fsu"/> Although it occasionally occurs in frequently burned old-field communities, it is more typical of native pine communities which have minimal soil disturbance.<ref name="fsu"/> It does not respond to soil disturbance by clearcutting and chopping in North Florida flatwoods forests.<ref>Moore, W.H., B.F. Swindel, and W.S. Terry. (1982). Vegetative Response to Clearcutting and Chopping in a North Florida Flatwoods Forest. Journal of Range Management 35(2):214-218.</ref> | + | ''C. mariana'' ranges from dry<ref name=wp> Walker, J. and R. K. Peet (1983). "Composition and species diversity of pine-wiregrass savannas of the Green Swamp, North Carolina." Vegetation 55: 163-179. </ref> to moist sand soils,<ref name="fsu"/> and thought it typically lives in high light areas, it can also live in partially shaded areas (54% ambient light conditions).<ref name=Cathey> Cathey, S. E., L. R. Boring, et al. (2010). "Assessment of N2 fixation capability of native legumes from the longleaf pine-wiregrass ecosystem." Environmental and Experimental Botany 67: 444-450. </ref><ref name="fsu"/> Although it occasionally occurs in frequently burned old-field communities, it is more typical of native pine communities which have minimal soil disturbance.<ref name="fsu"/> It does not respond to soil disturbance by clearcutting and chopping in North Florida flatwoods forests.<ref>Moore, W.H., B.F. Swindel, and W.S. Terry. (1982). Vegetative Response to Clearcutting and Chopping in a North Florida Flatwoods Forest. Journal of Range Management 35(2):214-218.</ref> ''C. mariana'' was found to be neutral in its short-term response to single mechanical soil disturbances, but was a decreaser in its long-term response following cessation of repeated soil disturbance.<ref name=Dixon>Dixon, C. M., K. M. Robertson, A. M. Reid and M. T. Rother. 2024. Mechanical soil disturbance in a pine savanna has multiyear effects on plant species composition. Ecosphere 15(2):e4759.</ref> |
''C. mariana'' is also a characteristic species of the shortleaf pine-oak-hickory community type.<ref name= "Clewell">Clewell, A. F. (2013). "Prior prevalence of shortleaf pine-oak-hickory woodlands in the Tallahassee red hills." Castanea 78(4): 266-276.</ref> | ''C. mariana'' is also a characteristic species of the shortleaf pine-oak-hickory community type.<ref name= "Clewell">Clewell, A. F. (2013). "Prior prevalence of shortleaf pine-oak-hickory woodlands in the Tallahassee red hills." Castanea 78(4): 266-276.</ref> | ||
Latest revision as of 09:12, 1 August 2024
| Clitoria mariana | |
|---|---|

| |
| photo by Gil Nelson | |
| Scientific classification | |
| Kingdom: | Plantae |
| Division: | Magnoliophyta - Flowering plants |
| Class: | Magnoliopsida – Dicotyledons |
| Order: | Fabales |
| Family: | Fabaceae ⁄ Leguminosae |
| Genus: | Clitoria |
| Species: | C. mariana |
| Binomial name | |
| Clitoria mariana L. | |
| |
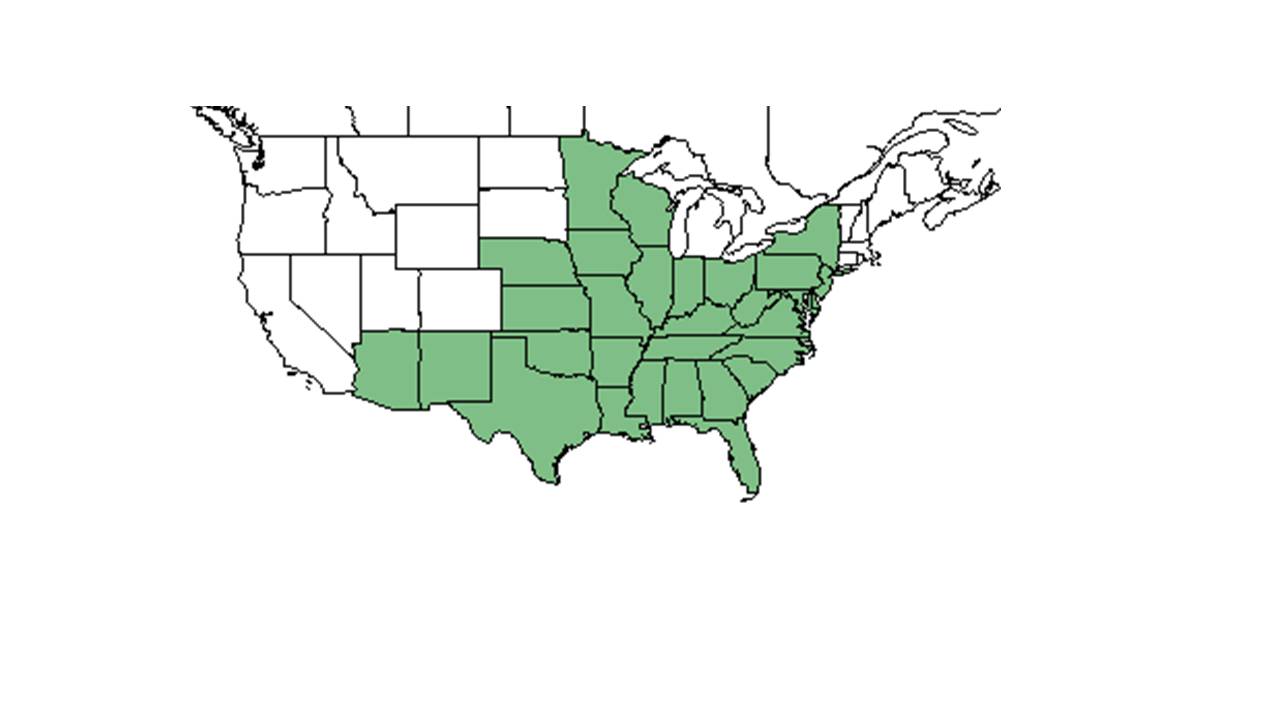
| Natural range of Clitoria mariana from USDA NRCS Plants Database. | |
Common names: Atlantic pigeonwings, butterfly pea, she-pea, Florida butterfly pea, Florida she-pea
Contents
Taxonomic notes
Synonym: none[1]
Varieties: Clitoria mariana Linnaeus var. mariana; Clitoria mariana Linnaeus var. pubescentia[1]
Description
Clitoria mariana is a trailing, twining, perennial, herbaceous vine growing up to 0.5 - 1 m long with smooth to short-pubescent stems. Some are large vines whereas other are small erect plants[2]. It is paraheliotropic, meaning the leaves are oriented parallel to incoming sunlight to reduce excessive light absorption.[3]The leaves are pinnately 3-foliolate with entire leaflets that are mostly ovate to lanceolate or ovate-oblong to somewhat elliptic, and grow 2 - 7 cm long; they are usually smooth or occasionally have short-pubescent on the underside. The stipules are ovate-lanceolate to lanceolate, ca. 2 - 4 mm long, tardily deciduous, and striate. The racemes are axillary, peduncles growing 0.5 - 4(6) cm long, usually shorter than subtending leaves, and have 1 - 3 flowers; pedicels are usually smooth or rarely short-pubescent, (2) 4 - 10 mm long, each subtended by a triangular to lanceolate, striate bract growing 1 - 3 mm long and with a pair of linear bractlets growing 3 - 6 mm long at or near the summits. The calyx is usually smooth or rarely short-pubescent and somewhat bilabiate. The tube is cylindric, growing 1 - 14 cm long, upper lobes are widely triangular, acute, growing 4 - 6 mm long, lateral lobes ovate-lanceolate, acuminate, growing 5 - 7 mm long, lowermost lobe lanceolate, acuminate, growing 6 - 8 mm long. The petals are pale blue or lavender in color, the standard are spurless, growing 4 - 6 cm long, and 3 - 4 cm wide. The wing petals are smaller and attached to the strongly incurved keel petals. The stamens have bundled filaments. The legumes are flattened, oblong-linear, growing 3 - 6 cm long; the stipe is elongated, growing 1 - 2 cm long, valves longitudinally twisting upon dehiscence. The seeds are sticky and adherent.[4]
This species can be commonly confused with Spurred Butterfly Pea (Centrosema virginianum), but the main difference between them is the upside down flowers and the banner pointing downward for Centrosema while Clitoria is erect.[5]
The root system of Clitoria mariana includes stem tubers which store non-structural carbohydrates (NSC) important for both resprouting following fire and persisting during long periods of fire exclusion.[6] Diaz-Toribio and Putz (2021) recorded this species to have an NSC concentration of 412.9 mg/g (ranking 4 out of 100 species studied) and water content of 69.5% (ranking 18 out of 100 species studied).[6]
According to Diaz-Torbio and Putz (2021), Clitoria mariana has stem tubers with a below-ground to above-ground biomass ratio of 0.74 and nonstructural carbohydrate concentration of 412.9 mg g-1.[7]
Distribution
C. mariana is native to a wide range of the United States, from New York south to Florida, northwest to Minnesota and Nebraska, and west to Arizona and New Mexico.[8] It is also native to South America. While C. mariana var. mariana is found in most of this distribution, C. mariana var. pubescentia is endemic to the central and southern peninsular Florida, and C. mariana var. orientalis is endemic to southeast Asia.[9]
Ecology
Habitat
C. mariana is listed as a facultative upland species, and mostly occurs in non-wetland areas such as frequently burned longleaf pine-turkey oak sandhills,[2] sand pine scrub (Entisols), [10] flatwoods (Spodosols) [11] upland longleaf pine-wiregrass communities (Ultisols), and in the margins of mixed hardwood communities[2] and loblolly pine plantations. [12], but this species can occasionally occur in wetlands.[8]
C. mariana ranges from dry[13] to moist sand soils,[2] and thought it typically lives in high light areas, it can also live in partially shaded areas (54% ambient light conditions).[14][2] Although it occasionally occurs in frequently burned old-field communities, it is more typical of native pine communities which have minimal soil disturbance.[2] It does not respond to soil disturbance by clearcutting and chopping in North Florida flatwoods forests.[15] C. mariana was found to be neutral in its short-term response to single mechanical soil disturbances, but was a decreaser in its long-term response following cessation of repeated soil disturbance.[16]
C. mariana is also a characteristic species of the shortleaf pine-oak-hickory community type.[17]
Associated species include Desmodium nudiflorum, Desmodium ochroleucum, Quercus laevis, Quercus stellata, Pinus palustris, Pinus echinata, Centrosema virginianum, Carya tomentosa, with other weeds, vines and trees in roadside ditches.[2]
Phenology
C. mariana has been observed flowering from May to September with peak inflorescence in July and fruits from September to October.[2][18] It is known to phenologically respond positively to disturbance, with increasing number of flowers produced after tornado damage in one study.[19]
Seed dispersal
This species is thought to be dispersed by translocation on animal fur or feathers.[20][21]
Fire ecology
Populations of Clitoria mariana have been known to persist through repeated annual burns,[22] and was found in Henley Park plots which were burned every one to two years in the winter.[11] It resprouts quickly after fire, which can be supported by the fact that it resprouted within a month after fire in Pavon's study. [23] It attained its peak in two-year rough plots at one study in Henley Park and another near Bainbridge GA, which are plots that had undergone two growing seasons since the last burn.[11][24] This is supported by Greenberg's study, which shows the peak percent cover to be 16 months after fire around 80% [10] As well, one study found C. mariana to establish at sites after a burn that was rare or absent before the fire.[25] In Kush's study, this species along with milk-pea became the dominant species proceeding plots that were burned in the winter.[26]
Herbivory and toxicology
Clitoria mariana is a game-food plant,[12] which means it is likely consumed by Gopherus polyphemus (Gopher tortoise) white-tailed deer, and bobwhite quail. [27] It consists between 5-10% of the diet of large mammals, and 2-5% of the diet for terrestrial birds.[28] C. mariana is especially important to northern bobwhite quail during the summer months.[29]
Conservation, cultivation, and restoration
C. mariana is listed as endangered by the New Jersey Department of Environmental Protection and Energy as well as the Pennsylvania Department of Conservation and Natural Resources.[8]
Cultural use
Photo Gallery
References and notes
- ↑ 1.0 1.1 Weakley, A.S. 2020. Flora of the Southeastern United States. Edition of 20 October 2020. University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: June 2014. Collectors: Loran C. Anderson, Wilson Baker, R.K. Godfrey, William Reese, Paul Redfearn, Robert L. Lazor, R. Kral, C. Jackson, O. Lakela, Paul R. Fantz, James R. Burkhalter, Andre F. Clewell, Travis MacClendon, Karen MacClendon, R. A. Norris, Rodie White, Kevin Oakes, Delzie Demaree, John W. Thieret, Alex Lasseigne, L. J. Uttal, D. S. Correll, H. B. Correll, Norlan C. Henderson, James D. Ray, Jr., Charles S. Wallis, Bayard Long, F. S. Earle, C. F. Baker, R. L. Wilbur, Mary E. Wharton, Raymond Athey, W.C. Coker, A. B. Seymour, A. E. Radford, and Rachel Williamson. States and Counties: Florida: Calhoun, Collier, Escambia, Flagler, Franklin, Gadsden, Hernando, Jackson, Jefferson, Lafayette, Leon, Liberty, Okaloosa, Taylor, Wakulla, and Walton. Georgia: Coffee, Grady, McIntosh, Pike, and Thomas. Alabama: Lee. North Carolina: Pamlico, Wake, and Wilson. Arkansas: Conway, Garland, Pulaski, and Saline. Missouri: Carter, Douglas, McDonald. Louisiana: Caddo, and Jackson. Virginia: Alleghany, Montgomery, and Sussex. Texas: Callahan, Morris, Upshur, and Van Zandt. Mississippi: Tishomingo. Oklahoma: Latimer. New Jersey: Cape May. Kentucky: Livingston, and Nelson. South Carolina: Darlington.
- ↑ KMR observation in July on Pebble Hill Plantation, Georgia.
- ↑ Radford, Albert E., Harry E. Ahles, and C. Ritchie Bell. Manual of the Vascular Flora of the Carolinas. 1964, 1968. The University of North Carolina Press. 636. Print.
- ↑ [[1]] Lady Bird Johnson Wildflower Center. Accessed: April 8, 2019
- ↑ 6.0 6.1 Diaz-Toribio, M.H. and F. E. Putz 2021. Underground carbohydrate stores and storage organs in fire-maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108: 432-442.
- ↑ Diaz‐Toribio, M. H. and F. E. Putz. 2021. Underground carbohydrate stores and storage organs in fire‐maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108(3):432-442.
- ↑ 8.0 8.1 8.2 USDA, NRCS. (2016). The PLANTS Database (http://plants.usda.gov, 8 April 2019). National Plant Data Team, Greensboro, NC 27401-4901 USA.
- ↑ Weakley, A. S. (2015). Flora of the Southern and Mid-Atlantic States. Chapel Hill, NC, University of North Carolina Herbarium.
- ↑ 10.0 10.1 Greenberg, C. H. (2003). "Vegetation recovery and stand structure following a prescribed stand-replacement burn in sand pine scrub." Natural Areas Journal 23: 141-151.
- ↑ 11.0 11.1 11.2 Brewer, J. S. and S. P. Cralle (2003). "Phosphorus addition reduces invasion of a longleaf pine savanna (southeastern USA) by a non-indigenous grass (Imperata cylindrica)." Plant Ecology 167: 237-245.
- ↑ 12.0 12.1 Cushwa, C. T. (1966). The response of herbaceous vegetation to prescribed burning. Asheville, USDA Forest Service.
- ↑ Walker, J. and R. K. Peet (1983). "Composition and species diversity of pine-wiregrass savannas of the Green Swamp, North Carolina." Vegetation 55: 163-179.
- ↑ Cathey, S. E., L. R. Boring, et al. (2010). "Assessment of N2 fixation capability of native legumes from the longleaf pine-wiregrass ecosystem." Environmental and Experimental Botany 67: 444-450.
- ↑ Moore, W.H., B.F. Swindel, and W.S. Terry. (1982). Vegetative Response to Clearcutting and Chopping in a North Florida Flatwoods Forest. Journal of Range Management 35(2):214-218.
- ↑ Dixon, C. M., K. M. Robertson, A. M. Reid and M. T. Rother. 2024. Mechanical soil disturbance in a pine savanna has multiyear effects on plant species composition. Ecosphere 15(2):e4759.
- ↑ Clewell, A. F. (2013). "Prior prevalence of shortleaf pine-oak-hickory woodlands in the Tallahassee red hills." Castanea 78(4): 266-276.
- ↑ Nelson, G. PanFlora: Plant data for the eastern United States with emphasis on the Southeastern Coastal Plains, Florida, and the Florida Panhandle. www.gilnelson.com/PanFlora/ Accessed: 7 DEC 2016
- ↑ Brewer, S. J., et al. (2012). "Do natural disturbances or the forestry practices that follow them convert forests to early-successional communities?" Ecological Applications 22: 442-458.
- ↑ Kirkman, L. Katherine. Unpublished database of seed dispersal mode of plants found in Coastal Plain longleaf pine-grasslands of the Jones Ecological Research Center, Georgia.
- ↑ Creech, M. N., et al. (2012). "Alteration and Recovery of Slash Pile Burn Sites in the Restoration of a Fire-Maintained Ecosystem." Restoration Ecology 20(4): 505-516.
- ↑ Robertson, K.M. Unpublished data collected from Pebble Hill Fire Plots, Pebble Hill Plantation, Thomasville, Georgia.
- ↑ Pavon, M. L. (1995). Diversity and response of ground cover arthropod communities to different seasonal burns in longleaf pine forests. Tallahassee, Florida A&M University.
- ↑ Buckner, J. L. and J. L. Landers (1979). "Fire and disking effects on herbaceous food plants and seed supplies." Journal of Wildlife Management 43: 807-811.
- ↑ Hutchinson, T. (2005). Fire and teh herbaceous layer of eastern oak forests. F. S. United States Department of Agriculture, Northern Research Station: 136-149.
- ↑ Kush, J. S., et al. (2000). Understory plant community response to season of burn in natural longleaf pine forests. Proceedings 21st Tall Timbers Fire Ecology Conference. Fire and forest ecology: innovative silviculture & vegetation management, Tallahassee, FL, Tall Timbers Research, Inc.
- ↑ Hainds, M. J., R. J. Mitchell, et al. (1999). "Distribution of native legumes (Leguminoseae) in frequently burned longleaf pine (Pinaceae)-wiregrass (Poaceae) ecosystems." American Journal of Botany 86: 1606-1614.
- ↑ Miller, J.H., and K.V. Miller. 1999. Forest plants of the southeast and their wildlife uses. Southern Weed Science Society.
- ↑ Jones, J. D. J. and M. J. Chamberlain (2004). "Efficacy of herbicides and fire to improve vegetative conditions for northern bobwhites in mature pine forests." Wildlife Society Bulletin 32: 1077-1084.