Difference between revisions of "Solidago odora"
(→Description) |
|||
| Line 17: | Line 17: | ||
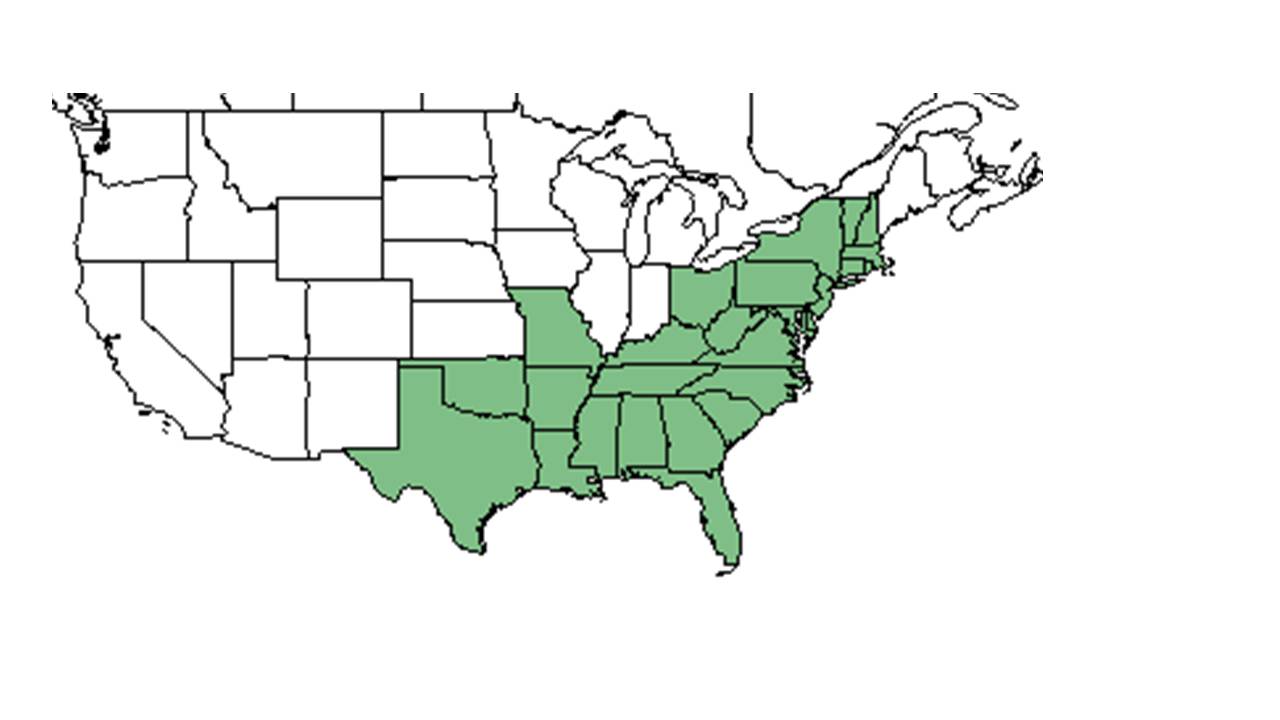
| range_map_caption = Natural range of ''Solidago odora'' from USDA NRCS [http://www.plants.usda.gov Plants Database]. | | range_map_caption = Natural range of ''Solidago odora'' from USDA NRCS [http://www.plants.usda.gov Plants Database]. | ||
}} | }} | ||
| + | |||
| + | Common name: anisescented goldenrod | ||
==Description== | ==Description== | ||
<!-- Basic life history facts such as annual/perrenial, monoecious/dioecious, root morphology, seed type, etc. --> | <!-- Basic life history facts such as annual/perrenial, monoecious/dioecious, root morphology, seed type, etc. --> | ||
| − | |||
==Distribution== | ==Distribution== | ||
| Line 25: | Line 26: | ||
===Habitat=== <!--Natural communities, human disturbed habitats, topography, hydrology, soils, light, fire regime requirements for removal of competition, etc.--> | ===Habitat=== <!--Natural communities, human disturbed habitats, topography, hydrology, soils, light, fire regime requirements for removal of competition, etc.--> | ||
It can be found on a forest edge or roadside (Boerner 1981). It is adapted to disturbance, including periodic burning (Boerner 1981). It can live in well-drained, acidic, sandy soils (Drewa et al 2006; Harrod et al 2000; Menges and Root 2004). It tolerates xeric conditions (Harrod et al 2000). | It can be found on a forest edge or roadside (Boerner 1981). It is adapted to disturbance, including periodic burning (Boerner 1981). It can live in well-drained, acidic, sandy soils (Drewa et al 2006; Harrod et al 2000; Menges and Root 2004). It tolerates xeric conditions (Harrod et al 2000). | ||
| − | It can be found in longleaf pine savannas (Drewa et al 2006). S. odora var. chapmanii is mostly found in sandhill and scrub communities (Menges and Root 2004). Found within the Coastal Plain (in Mississippi, for this study) where longleaf pine orginially occupied the area (Wahlenberg et al 1939). | + | It can be found in longleaf pine savannas (Drewa et al 2006). ''S. odora'' var. ''chapmanii'' is mostly found in sandhill and scrub communities (Menges and Root 2004). Found within the Coastal Plain (in Mississippi, for this study) where longleaf pine orginially occupied the area (Wahlenberg et al 1939). |
Found in flatwoods that are mesic, fire-maintained savannas or sparse woodlands with nutrient-poor soils, dominated by longleaf pine (Brewer et al 2003). | Found in flatwoods that are mesic, fire-maintained savannas or sparse woodlands with nutrient-poor soils, dominated by longleaf pine (Brewer et al 2003). | ||
===Phenology=== <!--Timing off flowering, fruiting, seed dispersal, and environmental triggers. Cite PanFlora website if appropriate: http://www.gilnelson.com/PanFlora/ --> | ===Phenology=== <!--Timing off flowering, fruiting, seed dispersal, and environmental triggers. Cite PanFlora website if appropriate: http://www.gilnelson.com/PanFlora/ --> | ||
| − | S. odora var. chapmanii often blooms in late summer and fall (July through October), though some bloom in spring (Menges and Root 2004). | + | ''S. odora'' var. ''chapmanii'' often blooms in late summer and fall (July through October), though some bloom in spring (Menges and Root 2004). |
===Seed dispersal=== | ===Seed dispersal=== | ||
It is a wind-dispersed species (Boerner 1981). | It is a wind-dispersed species (Boerner 1981). | ||
===Seed bank and germination=== | ===Seed bank and germination=== | ||
| − | S. odora var. chapmanii does not seem to form a large persistent seed bank (Menges and Root 2004). However, S. odora had a 12% germination rate in an experiment by Coffey and Kirkman in the second year after burial, showing that the seed bank can persist at least two years (2006). - Between fires, S. odora var. chapmanii can persist as suppressed ramets (a persistent bud bank), which gives can give it an advantage over competitors (Menges and Root 2004). | + | ''S. odora'' var. ''chapmanii'' does not seem to form a large persistent seed bank (Menges and Root 2004). However, S. odora had a 12% germination rate in an experiment by Coffey and Kirkman in the second year after burial, showing that the seed bank can persist at least two years (2006). - Between fires, ''S. odora'' var. ''chapmanii'' can persist as suppressed ramets (a persistent bud bank), which gives can give it an advantage over competitors (Menges and Root 2004). |
===Fire ecology=== <!--Fire tolerance, fire dependence, adaptive fire responses--> | ===Fire ecology=== <!--Fire tolerance, fire dependence, adaptive fire responses--> | ||
| − | It thrives in the years post-fire (Harrod et al 2000). Lewis and Harshbarger found out that S. odora responded positively to a wide variety of long-term burning treatments, but best to periodic summer and biennial summer burnings. S. odora was not present in the unburned control plot (1976). After fire, S. odora var. chapmanii can regenerate by seedlings, clonal ramets, or resprouting. It is thought that timing of fires may affect subsequent flowering. Flowering occurred abundantly in most plots during the year following fire, but experienced a marked decline afterwards (Menges and Root 2004). | + | It thrives in the years post-fire (Harrod et al 2000). Lewis and Harshbarger found out that S. odora responded positively to a wide variety of long-term burning treatments, but best to periodic summer and biennial summer burnings. S. odora was not present in the unburned control plot (1976). After fire, ''S. odora'' var. ''chapmanii'' can regenerate by seedlings, clonal ramets, or resprouting. It is thought that timing of fires may affect subsequent flowering. Flowering occurred abundantly in most plots during the year following fire, but experienced a marked decline afterwards (Menges and Root 2004). |
===Pollination=== | ===Pollination=== | ||
| − | + | The following Hymenoptera families and species were observed visiting flowers of ''Solidago odora'' at Archbold Biological Station (Deyrup 2015): | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | Apidae: ''Apis mellifera, Bombus impatiens, Nomada fervida, Xylocopa virginica krombeini'' | |
| − | + | Colletidae: ''Colletes mandibularis'' | |
| − | + | Halictidae: ''Agapostemon splendens, Augochlorella aurata, Augochloropsis metallica, A. sumptuosa, Halictus poeyi, Lasioglossum coreopsis, L. nymphalis, L. placidensis, Sphecodes heraclei'' | |
| − | + | Leucospidae: ''Leucospis slossonae, L. affinis, L. robertsoni, L. slossonae'' | |
| − | + | Megachilidae: ''Anthidiellum perplexus, Coelioxys sayi, Dianthidium floridiense, Dolichostelis louisiae, Megachile albitarsis, M. mendica, M. texana'' | |
| − | + | Pompilidae: ''Anoplius atrox, Paracyphonyx funereus'' | |
| − | + | Sphecidae: ''Ammophila urnaria, Bembix sayi, Bicyrtes capnoptera, B. quadrifasciata, Cerceris blakei, C. flavofasciata floridensis, C. fumipennis, Ectemnius decemmaculatus tequesta, Isodontia auripes, I. exornata, Oxybelus decorosum, Palmodes dimidiatus, Philanthus ventilabris, Prionyx thomae, Stictiella serrata, Tachytes grisselli, T. guatemalensis, T. pepticus, T. validus'' | |
| − | Vespidae: Zethus spinipes | + | Vespidae: ''Eumenes fraternus, E. smithii, Euodynerus boscii boharti, E. megaera, Pachodinerus erynnis, Pachodynerus erynnis, Parancistrocerus salcularis rufulus, Pseudodynerus quadrisectus, Stenodynerus fundatiformis, S. histrionalis rufustus, S. lineatifrons, S. oculeus, S. pulvinatus surrufus, Zethus spinipes'' |
===Use by animals=== <!--Herbivory, granivory, insect hosting, etc.--> | ===Use by animals=== <!--Herbivory, granivory, insect hosting, etc.--> | ||
| Line 168: | Line 65: | ||
Boerner, R. E. J. (1981). "Forest structure dynamics following wildfire and prescribed burning in the New Jersey pine barrens." American Midland Naturalist 105: 321-333. | Boerner, R. E. J. (1981). "Forest structure dynamics following wildfire and prescribed burning in the New Jersey pine barrens." American Midland Naturalist 105: 321-333. | ||
| − | + | Brewer, J. S. and S. P. Cralle (2003). "Phosphorus addition reduces invasion of a longleaf pine savanna (southeastern USA) by a non-indigenous grass (Imperata cylindrica)." Plant Ecology 167: 237-245. | |
Coffey, K. L. and L. K. Kirkman (2006). "Seed germination strategies of species with restoration potential in a fire-maintained pine savanna." Natural Areas Journal 26: 289-299. | Coffey, K. L. and L. K. Kirkman (2006). "Seed germination strategies of species with restoration potential in a fire-maintained pine savanna." Natural Areas Journal 26: 289-299. | ||
| + | |||
| + | Deyrup, M.A. and N.D. 2015. Database of observations of Hymenoptera visitations to flowers of plants on Archbold Biological Station, Florida, USA. | ||
Deyrup, M. and L. Deyrup (2012). "The diversity of insects visiting flowers of saw palmetto (Arecaceae)." Florida Entomologist 95(3): 711-730. | Deyrup, M. and L. Deyrup (2012). "The diversity of insects visiting flowers of saw palmetto (Arecaceae)." Florida Entomologist 95(3): 711-730. | ||
| + | |||
| + | Drewa, P. B., J. M. Thaxton, et al. (2006). "Responses of root-crown bearing shrubs to differences in fire regimes in Pinus palustris (Longleaf pine) savannas: exploring old-growth questions in second-growth systems." Applied Vegetation Science 9: 27-36. | ||
Harrod, J. C., M. E. Harmon, et al. (2000). "Post-fire succession and 20th century reduction in fire frequency on xeric southern Appalachian sites." Journal of Vegetation Science 11: 465-472. | Harrod, J. C., M. E. Harmon, et al. (2000). "Post-fire succession and 20th century reduction in fire frequency on xeric southern Appalachian sites." Journal of Vegetation Science 11: 465-472. | ||
| Line 178: | Line 79: | ||
Lewis, C. E. and T. J. Harshbarger (1976). "Shrub and herbaceous vegetation after 20 years of prescribed burning in the South Carolina coastal plain." Journal of Range Management 29: 13-18. | Lewis, C. E. and T. J. Harshbarger (1976). "Shrub and herbaceous vegetation after 20 years of prescribed burning in the South Carolina coastal plain." Journal of Range Management 29: 13-18. | ||
| − | + | Menges, E. S. and R. B. Root (2004). "The life of a fire-adapted Florida goldenrod, Solidago odora var. chapmanii." American Midland Naturalist 151: 65-78. | |
Wahlenberg, W. G., S. W. Greene, et al. (1939). Effects of fire and cattle grazing on longleaf pine lands as studied at McNeil Mississippi. Washington D.C., USDA. | Wahlenberg, W. G., S. W. Greene, et al. (1939). Effects of fire and cattle grazing on longleaf pine lands as studied at McNeil Mississippi. Washington D.C., USDA. | ||
| − | |||
| − | |||
Revision as of 19:27, 12 August 2015
| Solidago odora | |
|---|---|

| |
| Photo was taken by Gil Nelson | |
| Scientific classification | |
| Kingdom: | Plantae |
| Division: | Magnoliophyta – Flowering plants |
| Class: | Magnoliopsida – Dicotyledons |
| Order: | Asterales |
| Family: | Asteraceae ⁄ Compositae |
| Genus: | Solidago |
| Species: | S. odora |
| Binomial name | |
| Solidago odora Aiton | |
| |
| Natural range of Solidago odora from USDA NRCS Plants Database. | |
Common name: anisescented goldenrod
Contents
Description
Distribution
Ecology
Habitat
It can be found on a forest edge or roadside (Boerner 1981). It is adapted to disturbance, including periodic burning (Boerner 1981). It can live in well-drained, acidic, sandy soils (Drewa et al 2006; Harrod et al 2000; Menges and Root 2004). It tolerates xeric conditions (Harrod et al 2000). It can be found in longleaf pine savannas (Drewa et al 2006). S. odora var. chapmanii is mostly found in sandhill and scrub communities (Menges and Root 2004). Found within the Coastal Plain (in Mississippi, for this study) where longleaf pine orginially occupied the area (Wahlenberg et al 1939). Found in flatwoods that are mesic, fire-maintained savannas or sparse woodlands with nutrient-poor soils, dominated by longleaf pine (Brewer et al 2003).
Phenology
S. odora var. chapmanii often blooms in late summer and fall (July through October), though some bloom in spring (Menges and Root 2004).
Seed dispersal
It is a wind-dispersed species (Boerner 1981).
Seed bank and germination
S. odora var. chapmanii does not seem to form a large persistent seed bank (Menges and Root 2004). However, S. odora had a 12% germination rate in an experiment by Coffey and Kirkman in the second year after burial, showing that the seed bank can persist at least two years (2006). - Between fires, S. odora var. chapmanii can persist as suppressed ramets (a persistent bud bank), which gives can give it an advantage over competitors (Menges and Root 2004).
Fire ecology
It thrives in the years post-fire (Harrod et al 2000). Lewis and Harshbarger found out that S. odora responded positively to a wide variety of long-term burning treatments, but best to periodic summer and biennial summer burnings. S. odora was not present in the unburned control plot (1976). After fire, S. odora var. chapmanii can regenerate by seedlings, clonal ramets, or resprouting. It is thought that timing of fires may affect subsequent flowering. Flowering occurred abundantly in most plots during the year following fire, but experienced a marked decline afterwards (Menges and Root 2004).
Pollination
The following Hymenoptera families and species were observed visiting flowers of Solidago odora at Archbold Biological Station (Deyrup 2015):
Apidae: Apis mellifera, Bombus impatiens, Nomada fervida, Xylocopa virginica krombeini
Colletidae: Colletes mandibularis
Halictidae: Agapostemon splendens, Augochlorella aurata, Augochloropsis metallica, A. sumptuosa, Halictus poeyi, Lasioglossum coreopsis, L. nymphalis, L. placidensis, Sphecodes heraclei
Leucospidae: Leucospis slossonae, L. affinis, L. robertsoni, L. slossonae
Megachilidae: Anthidiellum perplexus, Coelioxys sayi, Dianthidium floridiense, Dolichostelis louisiae, Megachile albitarsis, M. mendica, M. texana
Pompilidae: Anoplius atrox, Paracyphonyx funereus
Sphecidae: Ammophila urnaria, Bembix sayi, Bicyrtes capnoptera, B. quadrifasciata, Cerceris blakei, C. flavofasciata floridensis, C. fumipennis, Ectemnius decemmaculatus tequesta, Isodontia auripes, I. exornata, Oxybelus decorosum, Palmodes dimidiatus, Philanthus ventilabris, Prionyx thomae, Stictiella serrata, Tachytes grisselli, T. guatemalensis, T. pepticus, T. validus
Vespidae: Eumenes fraternus, E. smithii, Euodynerus boscii boharti, E. megaera, Pachodinerus erynnis, Pachodynerus erynnis, Parancistrocerus salcularis rufulus, Pseudodynerus quadrisectus, Stenodynerus fundatiformis, S. histrionalis rufustus, S. lineatifrons, S. oculeus, S. pulvinatus surrufus, Zethus spinipes
Use by animals
Many herbivores, including certain species of beetles, moths, rodents, and rabbits, feed on S. odora var. chapmanii (Menges and Root 2004). Deyrup (2002) observed these bees, Colletes mandibularis, Perdita graenicheri, Agapostemon splendens, Augochlorella aurata, Augochloropsis metallica, A. sumptuosa, Diialictus coreopsis, D. nymphalis, D. placidensis, Halictus ligatus, Sphecodes heraclei, Dianthidium floridiense, Megachile albitarsis, M. mendica, M. texana, Apis mellifera, on Solidago odora var. chapmanii.
Diseases and parasites
Conservation and Management
Cultivation and restoration
Photo Gallery
References and notes
Boerner, R. E. J. (1981). "Forest structure dynamics following wildfire and prescribed burning in the New Jersey pine barrens." American Midland Naturalist 105: 321-333.
Brewer, J. S. and S. P. Cralle (2003). "Phosphorus addition reduces invasion of a longleaf pine savanna (southeastern USA) by a non-indigenous grass (Imperata cylindrica)." Plant Ecology 167: 237-245.
Coffey, K. L. and L. K. Kirkman (2006). "Seed germination strategies of species with restoration potential in a fire-maintained pine savanna." Natural Areas Journal 26: 289-299.
Deyrup, M.A. and N.D. 2015. Database of observations of Hymenoptera visitations to flowers of plants on Archbold Biological Station, Florida, USA.
Deyrup, M. and L. Deyrup (2012). "The diversity of insects visiting flowers of saw palmetto (Arecaceae)." Florida Entomologist 95(3): 711-730.
Drewa, P. B., J. M. Thaxton, et al. (2006). "Responses of root-crown bearing shrubs to differences in fire regimes in Pinus palustris (Longleaf pine) savannas: exploring old-growth questions in second-growth systems." Applied Vegetation Science 9: 27-36.
Harrod, J. C., M. E. Harmon, et al. (2000). "Post-fire succession and 20th century reduction in fire frequency on xeric southern Appalachian sites." Journal of Vegetation Science 11: 465-472.
Lewis, C. E. and T. J. Harshbarger (1976). "Shrub and herbaceous vegetation after 20 years of prescribed burning in the South Carolina coastal plain." Journal of Range Management 29: 13-18.
Menges, E. S. and R. B. Root (2004). "The life of a fire-adapted Florida goldenrod, Solidago odora var. chapmanii." American Midland Naturalist 151: 65-78.
Wahlenberg, W. G., S. W. Greene, et al. (1939). Effects of fire and cattle grazing on longleaf pine lands as studied at McNeil Mississippi. Washington D.C., USDA.