Difference between revisions of "Solidago odora"
(→Photo Gallery) |
ParkerRoth (talk | contribs) (→Description) |
||
| (58 intermediate revisions by 12 users not shown) | |||
| Line 18: | Line 18: | ||
}} | }} | ||
| − | Common | + | Common names: Anisescented goldenrod, Sweet goldenrod, Licorice goldenrod |
==Taxonomic notes== | ==Taxonomic notes== | ||
| − | ''S. odora | + | Synonyms: none |
| + | |||
| + | Varieties: ''Solidago odora'' var. ''odora'' | ||
| + | |||
| + | Subspecies: ''S. odora'' ssp. ''odora'' | ||
==Description== | ==Description== | ||
<!-- Basic life history facts such as annual/perrenial, monoecious/dioecious, root morphology, seed type, etc. --> | <!-- Basic life history facts such as annual/perrenial, monoecious/dioecious, root morphology, seed type, etc. --> | ||
A description of ''Solidago odora'' is provided in [http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=242417291 The Flora of North America]. | A description of ''Solidago odora'' is provided in [http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=242417291 The Flora of North America]. | ||
| + | |||
| + | The root system of ''Solidago odora'' includes corms which store non-structural carbohydrates (NSC) important for both resprouting following fire and persisting during long periods of fire exclusion.<ref name="Diaz"> Diaz-Toribio, M.H. and F. E. Putz 2021. Underground carbohydrate stores and storage organs in fire-maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108: 432-442.</ref>. Diaz-Toribio and Putz (2021) recorded this species to have an NSC concentration of 18.6 mg/g (ranking 98 out of 100 species studied) and water content of 50% (ranking 95 out of 100 species studied).<ref name="Diaz"/> | ||
| + | |||
| + | According to Diaz-Torbio and Putz (2021), ''Solidago odora'' has corms with a below-ground to above-ground biomass ratio of 1.04 and nonstructural carbohydrate concentration of 18.6 mg g<sup>-1</sup>.<ref>Diaz‐Toribio, M. H. and F. E. Putz. 2021. Underground carbohydrate stores and storage organs in fire‐maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108(3):432-442.</ref> | ||
==Distribution== | ==Distribution== | ||
| + | ''S. odora'' is found throughout the eastern United States from Texas to the east coast and north as far as southern Maine.<ref>Denhof, Carol. 2011. Understory Plant Spotlight Anise-scented Goldenrod Solidago odora Aiton. The Longleaf Leader. Vol. III. Iss. 3. Page 9</ref> | ||
| + | |||
==Ecology== | ==Ecology== | ||
===Habitat=== <!--Natural communities, human disturbed habitats, topography, hydrology, soils, light, fire regime requirements for removal of competition, etc.--> | ===Habitat=== <!--Natural communities, human disturbed habitats, topography, hydrology, soils, light, fire regime requirements for removal of competition, etc.--> | ||
| − | In the Coastal Plain region, ''S. odora'' can be found in sandhills, slashpine savannas, longleaf pine-scrub oak ridges, loblolly pine-sweetgum stands, longleaf pine-wiregrass sand ridges, depression marshes, edges of wetlands, sand dunes, live oak woodlands | + | In the Coastal Plain region, ''S. odora'' can be found in sandhills, slashpine savannas, longleaf pine-scrub oak ridges, loblolly pine-sweetgum stands, longleaf pine-wiregrass sand ridges, depression marshes, edges of wetlands, sand dunes, live oak woodlands,<ref name=fsu/> annually burned pinelands.<ref name=boe> Boerner, R. E. J. (1981). "Forest structure dynamics following wildfire and prescribed burning in the New Jersey pine barrens." American Midland Naturalist 105: 321-333.</ref><ref name=bre> Brewer, J. S. and S. P. Cralle (2003). "Phosphorus addition reduces invasion of a longleaf pine savanna (southeastern USA) by a non-indigenous grass (''Imperata cylindrica'')." Plant Ecology 167: 237-245.</ref><ref name=fsu>Florida State University Robert K. Godfrey Herbarium database. URL: [http://herbarium.bio.fsu.edu http://herbarium.bio.fsu.edu]. Last accessed: July 2015. Collectors: Travis MacClendon, Karen MacClendon, B. Boothe, M. Boothe, Bian Tan, Brenda Herring, Jame Amoroso, Loran C. Anderson, Gwynn W. Ramsey, R.K. Godfrey, R. S. Mitchell, Paul L. Redfearn, Jr., Angus Gholson, George R. Cooley, Richard J. Eaton, James D. Ray, Jr., R L Lazor, V. I. Sullivan, A. F. Clewell, R. Kral, H. E. Grelen, Gary R. Knight, R. A. Norris, R. Komarek, Cecil R Slaughter, S. W. Leonard, R. E. Perdue, Jr., Richard D. Houk, James D. Ray, Jr., Olga Lakela, Jackie Patman, Melanie R. Darst. States and Counties: Florida: Alachua, Calhoun, Clay, Columbia, Dixie, Escambia, Franklin, Gadsden, Gulf, Hernando, Highland, Hillsborough, Jackson, Jefferson, Leon, Liberty, Marion, Martin, Okaloosa, Osceola, Pasco, Pinellas, Polk, Santa Roasa, Seminole, St. Johns, St. Lucie, Suwannee, Taylor, Wakulla, Walton, Washington. Georgia: Camden, Grady, Thomas. Compiled by Tall Timbers Research Station and Land Conservancy.</ref> xeric areas,<ref name=har> Harrod, J. C., M. E. Harmon, et al. (2000). "Post-fire succession and 20th century reduction in fire frequency on xeric southern Appalachian sites." Journal of Vegetation Science 11: 465-472.</ref> longleaf pine savannas,<ref name=drewa> Drewa, P. B., J. M. Thaxton, et al. (2006). "Responses of root-crown bearing shrubs to differences in fire regimes in ''Pinus palustris'' (Longleaf pine) savannas: exploring old-growth questions in second-growth systems." Applied Vegetation Science 9: 27-36.</ref><ref name=fsu/> and scrub communities.<ref name=fsu/><ref name=mr04> Menges, E. S. and R. B. Root (2004). "The life of a fire-adapted Florida goldenrod, ''Solidago odora'' var. ''chapmanii''." American Midland Naturalist 151: 65-78.</ref> It can also be found in cut over fields, disturbed savannas, bulldozed pines, old fields, cut and slashed slash pine forests, vacant beach lots, cut over sandridges,<ref name=fsu/> and roadsides.<ref name=boe/><ref name=fsu/> Soils include sandy loam, loamy sand, sandy clay, red sandy clay, and sandy peat.<ref name=drewa/><ref name=fsu/> As well, it is considered to be indicative of non-agricultural history sites of frequently burned longleaf pine habitats.<ref name= "Hahn"/> ''Solidago odora'' is frequent and abundant in the Panhandle Xeric Sandhills community type and is an indicator species for the Clayhill Longleaf Woodlands community type as described in Carr et al. (2010).<ref>Carr, S.C., K.M. Robertson, and R.K. Peet. 2010. A vegetation classification of fire-dependent pinelands of Florida. Castanea 75:153-189.</ref> ''Solidago odora'' var. ''chapmanii'' is an indicator species for the Xeric Flathills community type as described in Carr et al. (2010).<ref>Carr, S.C., K.M. Robertson, and R.K. Peet. 2010. A vegetation classification of fire-dependent pinelands of Florida. Castanea 75:153-189.</ref> Additionally, ''S. odora'' has shown resistance to regrowth in reestablished coastal plains habitats that were disturbed by agriculture in South Carolina, making it a remnant woodlands indicator species.<ref name=brudvig>Brudvig, L.A., J.L. Orrock, E.I. Damschen, C.D. Collins, P.G. Hahn, W.B. Mattingly, J.W. Veldman, and J.L. Walker. (2014). Land-Use History and Contemporary Management Inform an Ecological Reference Model for Longleaf Pine Woodland Understory Plant Communities. PLoS ONE 9(1): e86604.</ref> |
| + | |||
| + | This species became absent in response to military training in west Georgia longleaf pine forests.<ref name=dale>Dale, V.H., S.C. Beyeler, and B. Jackson. (2002). Understory vegetation indicators of anthropogenic disturbance in longleaf pine forests at Fort Benning, Georgia, USA. Ecological Indicators 1(3):155-170.</ref> It also became absent in response to soil disturbance by agriculture in southwest Georgia.<ref name=hedman>Hedman, C.W., S.L. Grace, and S.E. King. (2000). Vegetation composition and structure of southern coastal plain pine forests: an ecological comparison. Forest Ecology and Management 134:233-247.</ref> | ||
| − | Associated species include ''Liatris, Panicum, Leptoloma cognata, Pityopsis graminifolia, Quercus minima, Q. laevis, Phyla nodiflora, Solidago puberula, Asclepias, Scutellaria floridana, Balduina, and Sporobolus'' | + | Associated species include ''Liatris, Panicum, Leptoloma cognata, Pityopsis graminifolia, Quercus minima, Q. laevis, Phyla nodiflora, Solidago puberula, Asclepias, Scutellaria floridana, Balduina, and Sporobolus.''<ref name=fsu/> |
===Phenology=== <!--Timing off flowering, fruiting, seed dispersal, and environmental triggers. Cite PanFlora website if appropriate: http://www.gilnelson.com/PanFlora/ --> | ===Phenology=== <!--Timing off flowering, fruiting, seed dispersal, and environmental triggers. Cite PanFlora website if appropriate: http://www.gilnelson.com/PanFlora/ --> | ||
| − | ''S. odora'' | + | ''S. odora'' has been observed to flower in January and March through November and fruit June through November.<ref name=fsu/><ref>Nelson, G. [http://www.gilnelson.com/ PanFlora]: Plant data for the eastern United States with emphasis on the Southeastern Coastal Plains, Florida, and the Florida Panhandle. www.gilnelson.com/PanFlora/ Accessed: 14 DEC 2016</ref> ''S. odora'' var. ''chapmanii'' often blooms in late summer and fall (July through October), though some bloom in spring.<ref name=mr04/> |
| − | |||
| − | ''S. odora'' var. ''chapmanii'' often blooms in late summer and fall (July through October), though some bloom in spring | ||
===Seed dispersal=== | ===Seed dispersal=== | ||
| − | + | This species is thought to be dispersed by wind.<ref> Kirkman, L. Katherine. Unpublished database of seed dispersal mode of plants found in Coastal Plain longleaf pine-grasslands of the Jones Ecological Research Center, Georgia.</ref> | |
===Seed bank and germination=== | ===Seed bank and germination=== | ||
| − | ''S. odora'' var. ''chapmanii'' does not seem to form a large persistent seed bank | + | ''S. odora'' var. ''chapmanii'' does not seem to form a large persistent seed bank.<ref name=mr04/> However, ''S. odora'' has been shown to persist in the seedbank for at least two years.<ref name=cof> Coffey, K. L. and L. K. Kirkman (2006). "Seed germination strategies of species with restoration potential in a fire-maintained pine savanna." Natural Areas Journal 26: 289-299. </ref> |
| + | |||
===Fire ecology=== <!--Fire tolerance, fire dependence, adaptive fire responses--> | ===Fire ecology=== <!--Fire tolerance, fire dependence, adaptive fire responses--> | ||
| − | + | Populations of ''Solidago odora'' have been known to persist through repeated annual burns,<ref>Robertson, K.M. Unpublished data collected from Pebble Hill Fire Plots, Pebble Hill Plantation, Thomasville, Georgia.</ref><ref>Glitzenstein, J. S., D. R. Streng, R. E. Masters, K. M. Robertson and S. M. Hermann 2012. Fire-frequency effects on vegetation in north Florida pinelands: Another look at the long-term Stoddard Fire Research Plots at Tall Timbers Research Station. Forest Ecology and Management 264: 197-209.</ref><ref>Platt, W.J., R. Carter, G. Nelson, W. Baker, S. Hermann, J. Kane, L. Anderson, M. Smith, K. Robertson. 2021. Unpublished species list of Wade Tract old-growth longleaf pine savanna, Thomasville, Georgia.</ref> and this species thrives in the years post-fire.<ref name=har/> Between fires, ''S. odora'' var. ''chapmanii'' can persist as suppressed ramets (a persistent bud bank), which can give it an advantage over competitors.<ref name=mr04/> ''S. odora'' has been shown to respond positively to a wide variety of long-term burning treatments, which the best responses to periodic summer and biennial summer burning.<ref name=lewis> Lewis, C. E. and T. J. Harshbarger (1976). "Shrub and herbaceous vegetation after 20 years of prescribed burning in the South Carolina coastal plain." Journal of Range Management 29: 13-18. </ref>''S. odora'' was not present in the unburned control plot of one experiment.<ref name=lewis/> After fire, ''S. odora'' var. ''chapmanii'' can regenerate by seed, clonal ramets, or resprouting.<ref name=mr04/> It is thought that timing of fires may affect subsequent flowering. Flowering occurred abundantly in most plots during the year following fire, but experienced a marked decline afterwards.<ref name=mr04/> | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | Pompilidae | + | <!--===Pollination===--> |
| + | ===Herbivory and toxicology===<!--Common herbivores, granivory, insect hosting, poisonous chemicals, allelopathy, etc--> | ||
| + | ''Solidago odora'' has been observed at the Archbold Biological Station to host bee species such as ''Colletes mandibularis'' (family Colletidae), bees from the Apidae family such as ''Apis mellifera, Bombus impatiens, Nomada fervida,'' and ''Xylocopa virginica krombeini'', bees from the Halictidae family such as ''Agapostemon splendens, Augochlorella aurata, Augochloropsis metallica, A. sumptuosa, Halictus poeyi, Lasioglossum coreopsis, L. nymphalis, L. placidensis,'' and ''Sphecodes heraclei'', bees and wasps from the Leucospidae family such as ''Leucospis slossonae, L. affinis, L. robertsoni,'' and ''L. slossonae'', bees from the Megachilidae family such as ''Anthidiellum perplexus, Coelioxys sayi, Dianthidium floridiense, Dolichostelis louisiae, Megachile albitarsis, M. mendica,'' and ''M. texana'', wasps from the Pompilidae family such as ''Anoplius atrox'' and ''Paracyphonyx funereus'', wasps from the Sphecidae family such as ''Ammophila urnaria, Bembix sayi, Bicyrtes capnoptera, B. quadrifasciata, Cerceris blakei, C. flavofasciata floridensis, C. fumipennis, Ectemnius decemmaculatus tequesta, Isodontia auripes, I. exornata, Oxybelus decorosum, Palmodes dimidiatus, Philanthus ventilabris, Prionyx thomae, Stictiella serrata, Tachytes grisselli, T. guatemalensis, T. pepticus,'' and ''T. validus'', wasps from the Vespidae family such as ''Eumenes fraternus, E. smithii, Euodynerus boscii boharti, E. megaera, Pachodinerus erynnis, Pachodynerus erynnis, Parancistrocerus salcularis rufulus, Pseudodynerus quadrisectus, Stenodynerus fundatiformis, S. histrionalis rufustus, S. lineatifrons, S. oculeus, S. pulvinatus surrufus,'' and ''Zethus spinipes''.<ref name=dey> Deyrup, M.A. and N.D. 2015. Database of observations of Hymenoptera visitations to flowers of plants on Archbold Biological Station, Florida, USA. </ref> Additionally, ''S. odora'' has been observed to host ''Uroleucon sp.'' (family Aphididae).<ref>Discoverlife.org [https://www.discoverlife.org/20/q?search=Bidens+albaDiscoverlife.org|Discoverlife.org]</ref> | ||
| − | + | Many herbivores, including certain species of beetles, moths, rodents, and rabbits, feed on ''S. odora'' var. ''chapmanii''.<ref name=mr04/> As well, common grasshoppers, including ''Melanopus angustipennis'' and grasshoppers in the subfamilies Melanoplinae and Cyrtacanthacridinae, were found to prefer ''Solidago odora'' compared to many other herbaceous species which may be due to the high nutrition in the plant. When exposed to herbivores, it was found to have an overall reduced biomass.<ref name= "Hahn">Hahn, P. C. and J. L. Orrock (2015). "Land-use legacies and present fire regimes interact to mediate herbivory by altering the neighboring plant community." Oikos 124: 497-506.</ref> Deyrup observed these bees, ''Colletes mandibularis, Perdita graenicheri, Agapostemon splendens, Augochlorella aurata, Augochloropsis metallica, A. sumptuosa, Diialictus coreopsis, D. nymphalis, D. placidensis, Halictus ligatus, Sphecodes heraclei, Dianthidium floridiense, Megachile albitarsis, M. mendica, M. texana, Apis mellifera, on Solidago odora'' var. ''chapmanii''.<ref name=dey02> Deyrup, M. and L. Deyrup (2012). "The diversity of insects visiting flowers of saw palmetto (Arecaceae)." Florida Entomologist 95(3): 711-730.</ref> | |
| + | <!--===Diseases and parasites===--> | ||
| − | + | ==Conservation, cultivation, and restoration== | |
| + | ''S. odora'' should avoid soil disturbance by agriculture and military training to conserve its presence in pine communities.<ref name=brudvig/><ref name=dale/><ref name=hedman/> | ||
| − | === | + | ==Cultural use== |
| − | + | The leaves can be dried and used as a substitute for Chinese teas.<ref> Fernald, et al. 1958. Edible Plants of Eastern North America. Harper and Row Publishers, New York.</ref> | |
| − | |||
| − | |||
| − | |||
| − | |||
==Photo Gallery== | ==Photo Gallery== | ||
<gallery widths=180px> | <gallery widths=180px> | ||
| + | File:Solidago odora (2).jpg| <center> ''Solidago odora'' root <p> Photo by Kevin Robertson </p> <p>Pebble Hill Plantation</p> <p>2015</p> | ||
</gallery> | </gallery> | ||
| − | |||
==References and notes== | ==References and notes== | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Latest revision as of 09:46, 3 July 2024
| Solidago odora | |
|---|---|

| |
| Photo was taken by Gil Nelson | |
| Scientific classification | |
| Kingdom: | Plantae |
| Division: | Magnoliophyta – Flowering plants |
| Class: | Magnoliopsida – Dicotyledons |
| Order: | Asterales |
| Family: | Asteraceae ⁄ Compositae |
| Genus: | Solidago |
| Species: | S. odora |
| Binomial name | |
| Solidago odora Aiton | |
| |
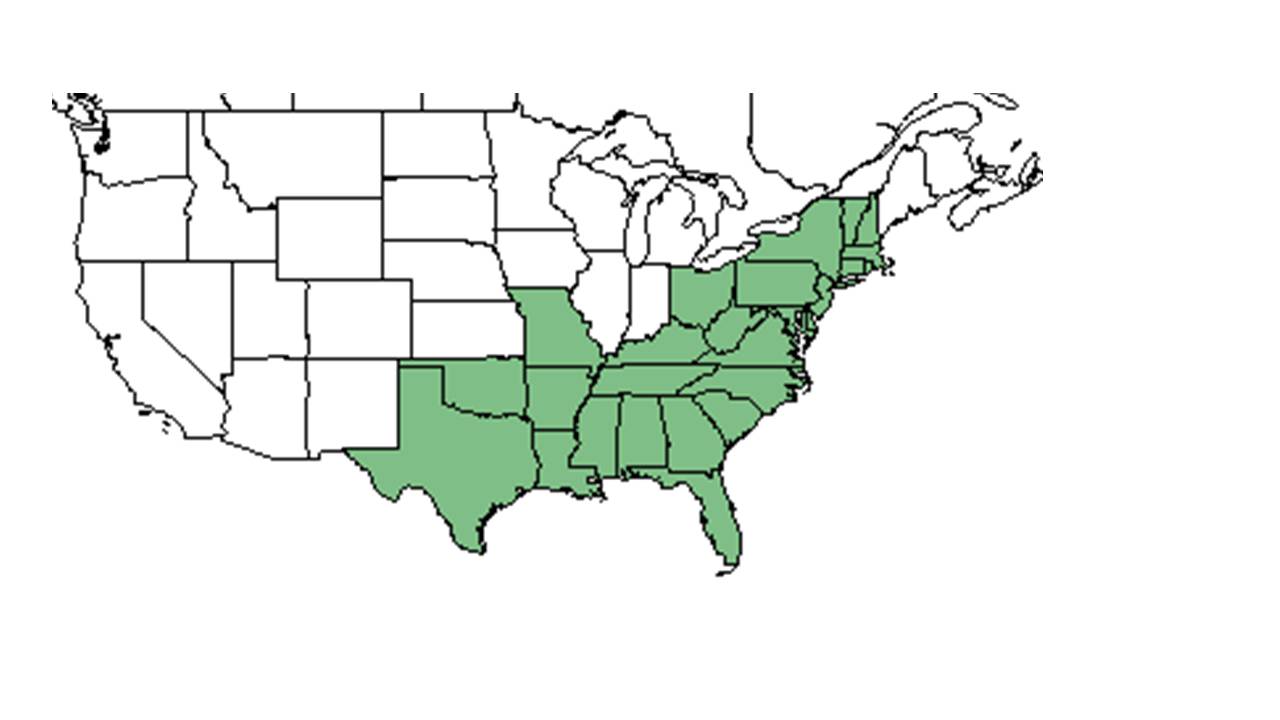
| Natural range of Solidago odora from USDA NRCS Plants Database. | |
Common names: Anisescented goldenrod, Sweet goldenrod, Licorice goldenrod
Contents
Taxonomic notes
Synonyms: none
Varieties: Solidago odora var. odora
Subspecies: S. odora ssp. odora
Description
A description of Solidago odora is provided in The Flora of North America.
The root system of Solidago odora includes corms which store non-structural carbohydrates (NSC) important for both resprouting following fire and persisting during long periods of fire exclusion.[1]. Diaz-Toribio and Putz (2021) recorded this species to have an NSC concentration of 18.6 mg/g (ranking 98 out of 100 species studied) and water content of 50% (ranking 95 out of 100 species studied).[1]
According to Diaz-Torbio and Putz (2021), Solidago odora has corms with a below-ground to above-ground biomass ratio of 1.04 and nonstructural carbohydrate concentration of 18.6 mg g-1.[2]
Distribution
S. odora is found throughout the eastern United States from Texas to the east coast and north as far as southern Maine.[3]
Ecology
Habitat
In the Coastal Plain region, S. odora can be found in sandhills, slashpine savannas, longleaf pine-scrub oak ridges, loblolly pine-sweetgum stands, longleaf pine-wiregrass sand ridges, depression marshes, edges of wetlands, sand dunes, live oak woodlands,[4] annually burned pinelands.[5][6][4] xeric areas,[7] longleaf pine savannas,[8][4] and scrub communities.[4][9] It can also be found in cut over fields, disturbed savannas, bulldozed pines, old fields, cut and slashed slash pine forests, vacant beach lots, cut over sandridges,[4] and roadsides.[5][4] Soils include sandy loam, loamy sand, sandy clay, red sandy clay, and sandy peat.[8][4] As well, it is considered to be indicative of non-agricultural history sites of frequently burned longleaf pine habitats.[10] Solidago odora is frequent and abundant in the Panhandle Xeric Sandhills community type and is an indicator species for the Clayhill Longleaf Woodlands community type as described in Carr et al. (2010).[11] Solidago odora var. chapmanii is an indicator species for the Xeric Flathills community type as described in Carr et al. (2010).[12] Additionally, S. odora has shown resistance to regrowth in reestablished coastal plains habitats that were disturbed by agriculture in South Carolina, making it a remnant woodlands indicator species.[13]
This species became absent in response to military training in west Georgia longleaf pine forests.[14] It also became absent in response to soil disturbance by agriculture in southwest Georgia.[15]
Associated species include Liatris, Panicum, Leptoloma cognata, Pityopsis graminifolia, Quercus minima, Q. laevis, Phyla nodiflora, Solidago puberula, Asclepias, Scutellaria floridana, Balduina, and Sporobolus.[4]
Phenology
S. odora has been observed to flower in January and March through November and fruit June through November.[4][16] S. odora var. chapmanii often blooms in late summer and fall (July through October), though some bloom in spring.[9]
Seed dispersal
This species is thought to be dispersed by wind.[17]
Seed bank and germination
S. odora var. chapmanii does not seem to form a large persistent seed bank.[9] However, S. odora has been shown to persist in the seedbank for at least two years.[18]
Fire ecology
Populations of Solidago odora have been known to persist through repeated annual burns,[19][20][21] and this species thrives in the years post-fire.[7] Between fires, S. odora var. chapmanii can persist as suppressed ramets (a persistent bud bank), which can give it an advantage over competitors.[9] S. odora has been shown to respond positively to a wide variety of long-term burning treatments, which the best responses to periodic summer and biennial summer burning.[22]S. odora was not present in the unburned control plot of one experiment.[22] After fire, S. odora var. chapmanii can regenerate by seed, clonal ramets, or resprouting.[9] It is thought that timing of fires may affect subsequent flowering. Flowering occurred abundantly in most plots during the year following fire, but experienced a marked decline afterwards.[9]
Herbivory and toxicology
Solidago odora has been observed at the Archbold Biological Station to host bee species such as Colletes mandibularis (family Colletidae), bees from the Apidae family such as Apis mellifera, Bombus impatiens, Nomada fervida, and Xylocopa virginica krombeini, bees from the Halictidae family such as Agapostemon splendens, Augochlorella aurata, Augochloropsis metallica, A. sumptuosa, Halictus poeyi, Lasioglossum coreopsis, L. nymphalis, L. placidensis, and Sphecodes heraclei, bees and wasps from the Leucospidae family such as Leucospis slossonae, L. affinis, L. robertsoni, and L. slossonae, bees from the Megachilidae family such as Anthidiellum perplexus, Coelioxys sayi, Dianthidium floridiense, Dolichostelis louisiae, Megachile albitarsis, M. mendica, and M. texana, wasps from the Pompilidae family such as Anoplius atrox and Paracyphonyx funereus, wasps from the Sphecidae family such as Ammophila urnaria, Bembix sayi, Bicyrtes capnoptera, B. quadrifasciata, Cerceris blakei, C. flavofasciata floridensis, C. fumipennis, Ectemnius decemmaculatus tequesta, Isodontia auripes, I. exornata, Oxybelus decorosum, Palmodes dimidiatus, Philanthus ventilabris, Prionyx thomae, Stictiella serrata, Tachytes grisselli, T. guatemalensis, T. pepticus, and T. validus, wasps from the Vespidae family such as Eumenes fraternus, E. smithii, Euodynerus boscii boharti, E. megaera, Pachodinerus erynnis, Pachodynerus erynnis, Parancistrocerus salcularis rufulus, Pseudodynerus quadrisectus, Stenodynerus fundatiformis, S. histrionalis rufustus, S. lineatifrons, S. oculeus, S. pulvinatus surrufus, and Zethus spinipes.[23] Additionally, S. odora has been observed to host Uroleucon sp. (family Aphididae).[24]
Many herbivores, including certain species of beetles, moths, rodents, and rabbits, feed on S. odora var. chapmanii.[9] As well, common grasshoppers, including Melanopus angustipennis and grasshoppers in the subfamilies Melanoplinae and Cyrtacanthacridinae, were found to prefer Solidago odora compared to many other herbaceous species which may be due to the high nutrition in the plant. When exposed to herbivores, it was found to have an overall reduced biomass.[10] Deyrup observed these bees, Colletes mandibularis, Perdita graenicheri, Agapostemon splendens, Augochlorella aurata, Augochloropsis metallica, A. sumptuosa, Diialictus coreopsis, D. nymphalis, D. placidensis, Halictus ligatus, Sphecodes heraclei, Dianthidium floridiense, Megachile albitarsis, M. mendica, M. texana, Apis mellifera, on Solidago odora var. chapmanii.[25]
Conservation, cultivation, and restoration
S. odora should avoid soil disturbance by agriculture and military training to conserve its presence in pine communities.[13][14][15]
Cultural use
The leaves can be dried and used as a substitute for Chinese teas.[26]
Photo Gallery
References and notes
- ↑ 1.0 1.1 Diaz-Toribio, M.H. and F. E. Putz 2021. Underground carbohydrate stores and storage organs in fire-maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108: 432-442.
- ↑ Diaz‐Toribio, M. H. and F. E. Putz. 2021. Underground carbohydrate stores and storage organs in fire‐maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108(3):432-442.
- ↑ Denhof, Carol. 2011. Understory Plant Spotlight Anise-scented Goldenrod Solidago odora Aiton. The Longleaf Leader. Vol. III. Iss. 3. Page 9
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: July 2015. Collectors: Travis MacClendon, Karen MacClendon, B. Boothe, M. Boothe, Bian Tan, Brenda Herring, Jame Amoroso, Loran C. Anderson, Gwynn W. Ramsey, R.K. Godfrey, R. S. Mitchell, Paul L. Redfearn, Jr., Angus Gholson, George R. Cooley, Richard J. Eaton, James D. Ray, Jr., R L Lazor, V. I. Sullivan, A. F. Clewell, R. Kral, H. E. Grelen, Gary R. Knight, R. A. Norris, R. Komarek, Cecil R Slaughter, S. W. Leonard, R. E. Perdue, Jr., Richard D. Houk, James D. Ray, Jr., Olga Lakela, Jackie Patman, Melanie R. Darst. States and Counties: Florida: Alachua, Calhoun, Clay, Columbia, Dixie, Escambia, Franklin, Gadsden, Gulf, Hernando, Highland, Hillsborough, Jackson, Jefferson, Leon, Liberty, Marion, Martin, Okaloosa, Osceola, Pasco, Pinellas, Polk, Santa Roasa, Seminole, St. Johns, St. Lucie, Suwannee, Taylor, Wakulla, Walton, Washington. Georgia: Camden, Grady, Thomas. Compiled by Tall Timbers Research Station and Land Conservancy.
- ↑ 5.0 5.1 Boerner, R. E. J. (1981). "Forest structure dynamics following wildfire and prescribed burning in the New Jersey pine barrens." American Midland Naturalist 105: 321-333.
- ↑ Brewer, J. S. and S. P. Cralle (2003). "Phosphorus addition reduces invasion of a longleaf pine savanna (southeastern USA) by a non-indigenous grass (Imperata cylindrica)." Plant Ecology 167: 237-245.
- ↑ 7.0 7.1 Harrod, J. C., M. E. Harmon, et al. (2000). "Post-fire succession and 20th century reduction in fire frequency on xeric southern Appalachian sites." Journal of Vegetation Science 11: 465-472.
- ↑ 8.0 8.1 Drewa, P. B., J. M. Thaxton, et al. (2006). "Responses of root-crown bearing shrubs to differences in fire regimes in Pinus palustris (Longleaf pine) savannas: exploring old-growth questions in second-growth systems." Applied Vegetation Science 9: 27-36.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 Menges, E. S. and R. B. Root (2004). "The life of a fire-adapted Florida goldenrod, Solidago odora var. chapmanii." American Midland Naturalist 151: 65-78.
- ↑ 10.0 10.1 Hahn, P. C. and J. L. Orrock (2015). "Land-use legacies and present fire regimes interact to mediate herbivory by altering the neighboring plant community." Oikos 124: 497-506.
- ↑ Carr, S.C., K.M. Robertson, and R.K. Peet. 2010. A vegetation classification of fire-dependent pinelands of Florida. Castanea 75:153-189.
- ↑ Carr, S.C., K.M. Robertson, and R.K. Peet. 2010. A vegetation classification of fire-dependent pinelands of Florida. Castanea 75:153-189.
- ↑ 13.0 13.1 Brudvig, L.A., J.L. Orrock, E.I. Damschen, C.D. Collins, P.G. Hahn, W.B. Mattingly, J.W. Veldman, and J.L. Walker. (2014). Land-Use History and Contemporary Management Inform an Ecological Reference Model for Longleaf Pine Woodland Understory Plant Communities. PLoS ONE 9(1): e86604.
- ↑ 14.0 14.1 Dale, V.H., S.C. Beyeler, and B. Jackson. (2002). Understory vegetation indicators of anthropogenic disturbance in longleaf pine forests at Fort Benning, Georgia, USA. Ecological Indicators 1(3):155-170.
- ↑ 15.0 15.1 Hedman, C.W., S.L. Grace, and S.E. King. (2000). Vegetation composition and structure of southern coastal plain pine forests: an ecological comparison. Forest Ecology and Management 134:233-247.
- ↑ Nelson, G. PanFlora: Plant data for the eastern United States with emphasis on the Southeastern Coastal Plains, Florida, and the Florida Panhandle. www.gilnelson.com/PanFlora/ Accessed: 14 DEC 2016
- ↑ Kirkman, L. Katherine. Unpublished database of seed dispersal mode of plants found in Coastal Plain longleaf pine-grasslands of the Jones Ecological Research Center, Georgia.
- ↑ Coffey, K. L. and L. K. Kirkman (2006). "Seed germination strategies of species with restoration potential in a fire-maintained pine savanna." Natural Areas Journal 26: 289-299.
- ↑ Robertson, K.M. Unpublished data collected from Pebble Hill Fire Plots, Pebble Hill Plantation, Thomasville, Georgia.
- ↑ Glitzenstein, J. S., D. R. Streng, R. E. Masters, K. M. Robertson and S. M. Hermann 2012. Fire-frequency effects on vegetation in north Florida pinelands: Another look at the long-term Stoddard Fire Research Plots at Tall Timbers Research Station. Forest Ecology and Management 264: 197-209.
- ↑ Platt, W.J., R. Carter, G. Nelson, W. Baker, S. Hermann, J. Kane, L. Anderson, M. Smith, K. Robertson. 2021. Unpublished species list of Wade Tract old-growth longleaf pine savanna, Thomasville, Georgia.
- ↑ 22.0 22.1 Lewis, C. E. and T. J. Harshbarger (1976). "Shrub and herbaceous vegetation after 20 years of prescribed burning in the South Carolina coastal plain." Journal of Range Management 29: 13-18.
- ↑ Deyrup, M.A. and N.D. 2015. Database of observations of Hymenoptera visitations to flowers of plants on Archbold Biological Station, Florida, USA.
- ↑ Discoverlife.org [1]
- ↑ Deyrup, M. and L. Deyrup (2012). "The diversity of insects visiting flowers of saw palmetto (Arecaceae)." Florida Entomologist 95(3): 711-730.
- ↑ Fernald, et al. 1958. Edible Plants of Eastern North America. Harper and Row Publishers, New York.
