Difference between revisions of "Balduina angustifolia"
(→Ecology) |
(→Description) |
||
| Line 28: | Line 28: | ||
The genus ''Balduina'' is characterized by receptacular bractlets connected in a honeycomb like structure surrounding the achene.<ref>Small, J.K. 1933. Manual of the Southeaster flora. New York. | The genus ''Balduina'' is characterized by receptacular bractlets connected in a honeycomb like structure surrounding the achene.<ref>Small, J.K. 1933. Manual of the Southeaster flora. New York. | ||
| − | + | </ref> | |
| − | ''Balduina angustifolia'' does not have specialized underground storage units apart from its fibrous roots.<ref name="Diaz"> Diaz-Toribio, M.H. and F. E. Putz 2021. Underground carbohydrate stores and storage organs in fire-maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108: 432-442.</ref> Diaz-Toribio and Putz (2021) recorded this species to have a water content of 65.5% (ranking 46 out of 100 species studied).<ref name="Diaz"/> | + | ''Balduina angustifolia'', specifically, is a biennial species that forms a basal rosette the first year and a leafy flowering stem the second year<ref name="Anderson">Anderson, R. C. and E. S. Menges (1997). "Effects of fire on sandhill herbs: nutrients, mycorrhizae, and biomass allocation." American Journal of Botany 84: 938-948.</ref>. The plant can grow up to 4 - 5 feet in height with many side branches near the top<ref name="Native">[[http://hawthornhillwildflowers.blogspot.com/2010/11/honeycombhead-balduina-angustifolia.html Native Florida Wildflowers]] Accessed December 1, 2015</ref>, and it does not have specialized underground storage units apart from its fibrous roots.<ref name="Diaz"> Diaz-Toribio, M.H. and F. E. Putz 2021. Underground carbohydrate stores and storage organs in fire-maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108: 432-442.</ref> Diaz-Toribio and Putz (2021) recorded this species to have a water content of 65.5% (ranking 46 out of 100 species studied).<ref name="Diaz"/> |
==Distribution== | ==Distribution== | ||
Revision as of 12:21, 23 June 2021
| Balduina angustifolia | |
|---|---|

| |
| Photo by Karan A. Rawlins, University of Georgia, Bugwood.org | |
| Scientific classification | |
| Kingdom: | Plantae |
| Division: | Magnoliophyta - Flowering plants |
| Class: | Magnoliopsida - Dicotyledons |
| Order: | Asterales |
| Family: | Asteraceae ⁄ Compositae |
| Genus: | Balduina |
| Species: | B. angustifolia |
| Binomial name | |
| Balduina angustifolia (Pursh) B.L. Rob. | |
| |
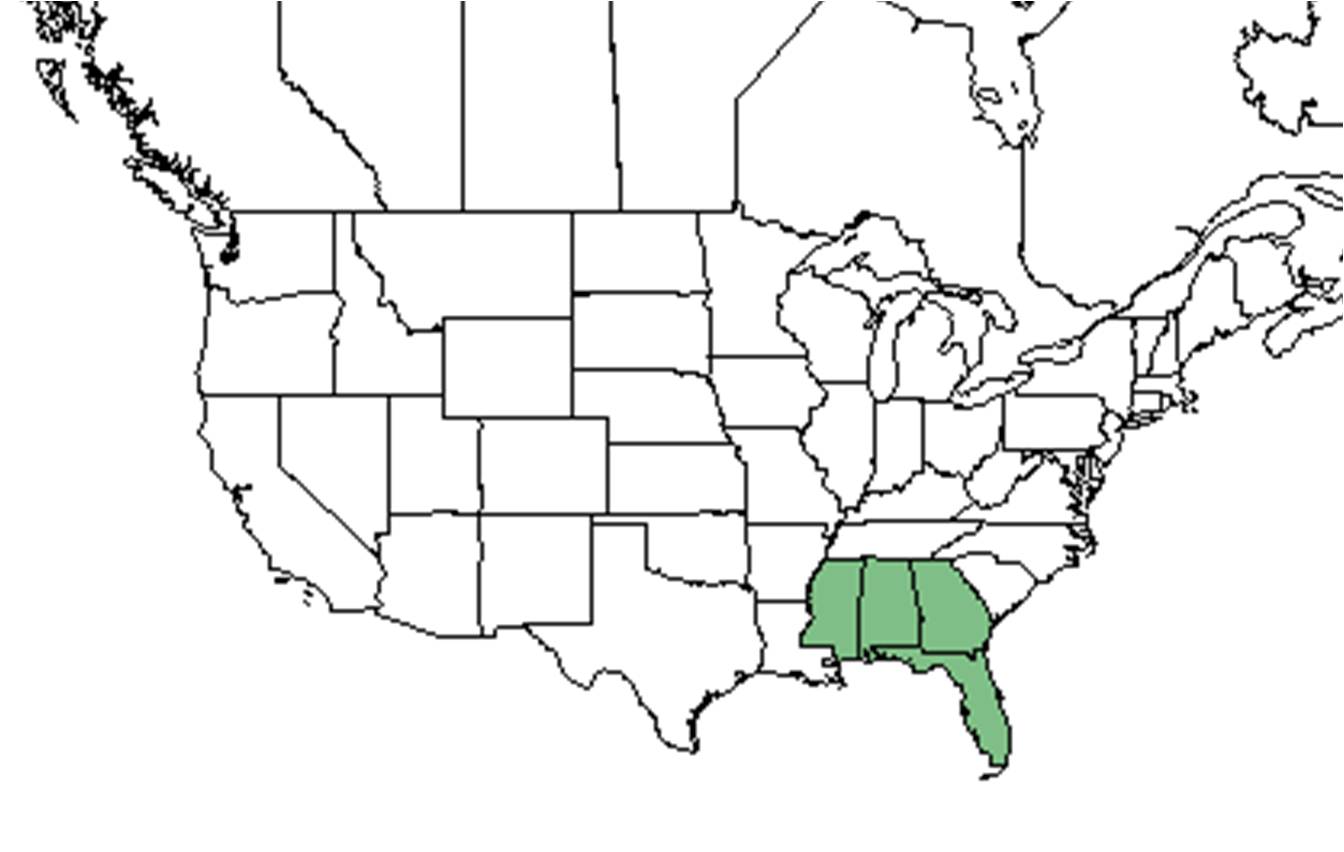
| Natural range of Balduina angustifolia from USDA NRCS Plants Database. | |
Common name: Coastal plain honeycombhead
Contents
Taxonomic notes
Synonyms: Actinospermum angustifolium (Pursh) Torrey & A. Gray.[1]
Varieties: none.[1]
Description
A description of Balduina angustifolia is provided in The Flora of North America.
The genus Balduina is characterized by receptacular bractlets connected in a honeycomb like structure surrounding the achene.[2]
Balduina angustifolia, specifically, is a biennial species that forms a basal rosette the first year and a leafy flowering stem the second year[3]. The plant can grow up to 4 - 5 feet in height with many side branches near the top[4], and it does not have specialized underground storage units apart from its fibrous roots.[5] Diaz-Toribio and Putz (2021) recorded this species to have a water content of 65.5% (ranking 46 out of 100 species studied).[5]
Distribution
The Balduina genera is endemic to the longleaf pine range from southeastern Virginia to central Florida and west to southeast Texas.[6]
Ecology
Habitat
In the Coastal Plain in Florida, B. angustifolia has been found in sand dunes; turkey oak sand ridges; pine scrubs; rosemary-oak scrubs; scrub oak-longleaf pine ridges; wiregrass/longleaf pine sandhills; pine flatwoods; bordering sidestreams; open woodlands; and xeric oak/saw palmetto scrubs.[7] There are greater populations of B. angustifolia in bare, open sands than in sites with shrubs or litter, making it a gap specialist.[8] It is early successional species and has been found to have a greater population growth in degraded scrubs compared to intact scrubs.[8] In a study conducted by Petru and Menges (2004) found that B. angustifolia responded to an experimental sand removal by elongating the flowering stems. It has been found in disturbed areas such as roadsides, highways, powerline corridors, bulldozed scrub oak ridges and clobbered slash pine forests.[7][9] Soil types include deep sand and loamy sand.[7] Associated species include Gaillardia, Pityopsis, Polygonella, Chrysoma, Ceratiola ericoides, Liatris, Leptoloma cognata, Pinus clausa, and Quercus virginiana.[7]
Sandhill and scrub habitats have a low availability of inorganic nutrients such as phosphorous causing B. angustifolia to depend on mycorrhizal fungi colonization on the taproot system.[3]
Balduina angustifolia is an indicator species for the Peninsula Xeric Sandhills community type as described in Carr et al. (2010).[10]
Phenology
Both the disc and ray flowers are yellow, with the ripe disc flowers forming a grey honeycomb shape seed.[4] The ripe seed can remain attached to the stem for months, allowing for favorable germination conditions in late winter and early spring.[4] It has been observed flowering August through November, with its peak inflorescence in October, and fruiting September and October.[7][11]
Fire ecology
Following a fire, the first year rosettes were unable to survive or resprout, making it a fire-sensitive biennial.[3] However, populations have been found to later re-establish on a burn site from seed.[3]
Pollination and use by animals
Balduina angustifolia has been observed at the Archbold Biological Station to be visited by ground-nesting bees such as Andrena fulvipennis (family Andrenidae), bees from the Apidae family such as Apis mellifera, Bombus impatiens, B. pennsylvanicus, Nomada fervida, Svastra aegis and Triepiolus concavus, sweat bees from the Halictidae family such as Agapostemon splendens, Augochlora pura, Augochlorella aurata, Augochloropsis metallica, A. sumptuosa, Dieunomia heteropoda, Halictus poeyi, Lasioglossum coreopsis, L. miniatulus, L. nymphalis. L. pectoralis and L. puteulanum, leafcutting bees from the Megachilidae family such as Anthidiellum perplexum, Coelioxys dolichos, C. germana, C. mexicana, C. modesta, C. sayi, C. texana, Dolichostelis louisae, Megachile albitarsis, M. pseudobrevis, M. georgica, M. inimica, M. mendica, M. petulans, M. policaris, M. pruina, M. texana, M. xylocopoides and Trachusa fontemvitae, thread-waisted wasps from the Sphecidae family such as Bembix sayi, Bicyrtes capnoptera, Ectemnius rufipes ais and Philanthus ventilabris, and wasps from the Vespidae family such as Mischocyttarus cubensis, Pachodynerus erynnis and Zethus slossonae.[12] Additionally, this species has been observed with ground-nesting bees from the Andrenidae family such as Perdita bequaerti and P. consobrina, as well as the bee Svastra aegis (family Apidae), platerer bees such as Colletes thysanellae (family Colletidae), and bees from the Melittidae family such as Hesperapis oraria.[13]
It is common in areas of soil disturbance produced by gopher tortoises.[3]
Conservation, cultivation, and restoration
Global conservation status: G5.[14]
Cultural use
Photo Gallery
References and notes
- ↑ 1.0 1.1 Weakley, A.S. 2015. Flora of the southern and mid-atlantic states. Working Draft of 21 May 2015. University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
- ↑ Small, J.K. 1933. Manual of the Southeaster flora. New York.
- ↑ 3.0 3.1 3.2 3.3 3.4 Anderson, R. C. and E. S. Menges (1997). "Effects of fire on sandhill herbs: nutrients, mycorrhizae, and biomass allocation." American Journal of Botany 84: 938-948.
- ↑ 4.0 4.1 4.2 [Native Florida Wildflowers] Accessed December 1, 2015
- ↑ 5.0 5.1 Diaz-Toribio, M.H. and F. E. Putz 2021. Underground carbohydrate stores and storage organs in fire-maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108: 432-442.
- ↑ Sorrie, B. A. and A. S. Weakley 2001. Coastal Plain valcular plant endemics: Phytogeographic patterns. Castanea 66: 50-82.
- ↑ 7.0 7.1 7.2 7.3 7.4 Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: October 2015. Collectors: William P. Adams, Jame Amoroso, Loran C. Anderson, Robert Blaisdell, K.E. Blum, A.F. Clewell, George R. Cooley, Richard J. Eaton, R.K. Godfrey, H.E. Grelen, Bruce Hansen, JoAnn Hansen, Richard D. Houk, R. Kral, Bob Lazor, S.W. Leonard, Sidney McDaniel, Thomas E. Miller, J.B. Morrill, Putnam, James D. Ray Jr., Siri von Reis, Paul L. Redfearn Jr., Paul O. Schallert, Victoria I. Sullivan, Bian Tan, E.L. Tyson, Jean Wooten. States and Counties: Florida: Bay, Broward, Calhoun, Collier, Columbia, Clay, Columbia, Dixie, Escambia, Franklin, Hernando, Highlands, Jackson, Lafayette, Lee, Levy, Liberty, Madison, Manatee, Martin, Okaloosa, Osceola, Pinellas, Polk, Putnam, Santa Rosa, Sarasota, Seminole, Suwannee, Taylor, Walton, Wakulla. Compiled by Tall Timbers Research Station and Land Conservancy.
- ↑ 8.0 8.1 Stephens, E. L., M. R. Tye, et al. (2014). "Habitat and microsite influence demography of two herbs in intact and degraded scrub." Population Ecology 56(3): 447-461.
- ↑ Petru, M. and E. S. Menges (2004). "Shifting sands in Florida scrub gaps and roadsides: Dynamic microsites for herbs." American Midland Naturalist 151(1): 101-113.
- ↑ Carr, S.C., K.M. Robertson, and R.K. Peet. 2010. A vegetation classification of fire- dependent pinelands of Florida. Castanea 75:153-189.
- ↑ Nelson, G. PanFlora: Plant data for the eastern United States with emphasis on the Southeastern Coastal Plains, Florida, and the Florida Panhandle. www.gilnelson.com/PanFlora/ Accessed: 7 DEC 2016
- ↑ Deyrup, M.A. 2015. Database of observations of Hymenoptera visitations to flowering plants on Archbold Biological Station, Florida, USA.
- ↑ Discoverlife.org [1]
- ↑ [NatureServe] Accessed: December 1, 2015