Difference between revisions of "Chamaecrista fasciculata"
(→Description) |
Adam.Vansant (talk | contribs) |
||
| (64 intermediate revisions by 13 users not shown) | |||
| Line 17: | Line 17: | ||
| range_map_caption = Natural range of ''Chamaecrista fasciculata'' from USDA NRCS [http://www.plants.usda.gov/core/profile?symbol=CHFA2 Plants Database]. | | range_map_caption = Natural range of ''Chamaecrista fasciculata'' from USDA NRCS [http://www.plants.usda.gov/core/profile?symbol=CHFA2 Plants Database]. | ||
}} | }} | ||
| − | Common name: partridge pea | + | Common name: Partridge Pea, common partridge-pea, tidal-marsh partridge pea |
==Taxonomic notes== | ==Taxonomic notes== | ||
| − | + | Synonyms: ''Chamaecrista puberula'' Greene; ''Cassia fasciculata'' var. ''brachiata'' (Pollard) Pullen ex Isely; ''Chamaecrista brachiata'' Pollard; ''Cassia fasciculata'' var. ''macrosperma'' Fernald<ref name=weakley>Weakley, A.S. 2020. Flora of the Southeastern United States. Edition of 20 October 2020. University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.</ref> | |
| − | |||
| − | |||
| − | + | Varieties: ''Chamaecrista fasciculata'' (Michaux) Greene ''var. 1''; ''Chamaecrista fasciculata'' (Michaux) Greene '' var. brachiata'' (Pollard) Isely; ''Chamaecrista fasciculata'' (Michaux) Greene var. ''fasciculata''; ''Chamaecrista fasciculata'' (Michaux) Greene var. ''macrosperma'' (Fernald) C.F. Reed; ''Chamaecrista littoralis'' Pollard; ''Chamaecrista mississippiensis'' (Pollard) Pollard ex Heller; ''Cassia fasciculata'' var. ''littoralis'' (Pollard) J.F. Macbride; ''Cassia fasciculata'' var. ''robusta'' (Pollard) J.F. Macbride; ''Chamaecrista robusta'' Pollard<ref name=weakley/> | |
| + | |||
| + | ==Description==<!-- Basic life history facts such as annual/perrenial, monoecious/dioecious, root morphology, seed type, etc. --> | ||
| + | |||
| + | |||
| + | ''Chameacrista fasciculata'' is an annual herb, growing 1.5-6 dm tall from the taproot. The stems and branches are glabrous to more commonly densely puberulent with incurved trichomes and occasionally also with villous trichomes to 2 mm long. The leaves are sensitive with a sessile, depressed, saucer-shaped gland, 0.5-1.5 mm in diam. near the middle of the petiole. Leaflets 12-36, linear-oblong, 1-2.5 cm long, 2-6 mm wide, inequilateral; stipules persistent, striate. Inflorescence are 1-6 flowered axillary fascicle. Pedicels grow up to 1-2 cm long. Sepals are lanceolate in shape, growing 9-12 mm long, and are acute. Petals are bright yellow in color, almost equal, growing 1-2 cm long; stamens 10, unequal, growing 10-13 mm long. The legume are elastically dehiscent, growing 3-7 cm long, and 5-7 mm broad, and are glabrate or appressed-puberulent to villous.<ref name=Radford> Radford, Albert E., Harry E. Ahles, and C. Ritchie Bell. Manual of the Vascular Flora of the Carolinas. 1964, 1968. The University of North Carolina Press. 577-8. Print.</ref> | ||
| + | |||
| + | ''Chameacrista fasciculata'' does not have specialized underground storage units apart from its taproot.<ref name="Diaz"> Diaz-Toribio, M.H. and F. E. Putz 2021. Underground carbohydrate stores and storage organs in fire-maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108: 432-442.</ref> Diaz-Toribio and Putz (2021) recorded this species to have an non-structural carbohydrate concentration of 76.2 mg/g (ranking 59 out of 100 species studied) and water content of 44% (ranking 35 out of 100 species studied).<ref name="Diaz"/> | ||
| + | |||
| + | According to Diaz-Torbio and Putz (2021), ''Chamaecrista fasiculata'' has taproots with a below-ground to above-ground biomass ratio of 0.14 and nonstructural carbohydrate concentration of 76.2 mg g<sup>-1</sup>.<ref>Diaz‐Toribio, M. H. and F. E. Putz. 2021. Underground carbohydrate stores and storage organs in fire‐maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108(3):432-442.</ref> | ||
==Distribution== | ==Distribution== | ||
| + | ''C. fasciculata'' is native to the eastern United States, excluding Vermont, New Hampshire, and Maine, west to New Mexico, South Dakota, and Minnesota.<ref name= "USDA">USDA, NRCS. (2016). The PLANTS Database (http://plants.usda.gov, 4 April 2019). National Plant Data Team, Greensboro, NC 27401-4901 USA.</ref> | ||
| + | |||
==Ecology== | ==Ecology== | ||
| + | Like other species in the Fabaceae family, ''C. fasciculata'' is a nitrogen-fixing plant that depends on microorganisms to help produce nitrogen compounds necessary for the plant's survival. This is conducted through a symbiosis of microorganisms inhabiting root nodules of the plant to give the plant direct access and the microorganisms a safe habitat.<ref name= "lady bird">[[https://www.wildflower.org/plants/search.php?search_field=&newsearch=true]] Lady Bird Johnson Wildflower Center. Accessed: April 4, 2019</ref> | ||
| + | |||
===Habitat=== <!--Natural communities, human disturbed habitats, topography, hydrology, soils, light, fire regime requirements for removal of competition, etc.--> | ===Habitat=== <!--Natural communities, human disturbed habitats, topography, hydrology, soils, light, fire regime requirements for removal of competition, etc.--> | ||
| + | ''C. fasciculata'' is a facultative upland species<ref name= "USDA"/> and can be found in sandy savannas of the Gulf Coastal Plain, and bluffs, prairies, river bottoms and banks, and upland woods of the Great Plains region. Generally, this species prefers well-drained and moderately lime souls such as sandy to sandy loam soils, but the wide range of soils include slightly acidic to moderately alkaline soils.<ref name= "Houck"/><ref name= "fact sheet"> USDA NRCS Plant Materials Program and J. M. Row. (2006). Plant Fact Sheet: Showy Partridge Pea ''Chamaecrista fasciculata''.N.R.C.S. United States Department of Agriculture. Manhattan, KS.</ref> ''C. fasciculata'' is also a common colonizer of disturbed areas<ref name= "Houck"/> can be found growing on recently abandoned gopher tortoise (''Gopherus polyphemus'') mounds,<ref name= "Kaczor">Kaczor, S. A. and D. C. Hartnett (1990). "Gopher tortoise (Gopherus polyphemus) effects on soils and vegetation in a Florida sandhill." American Midland Naturalist 123: 100-111.</ref>, dry sand, roadsides and other disturbed areas, sand ridges, sand dunes, upland old fields, scrub clearings, pine flatwoods, sandhills, wooded ravines, salt marshes, prairies, and edges of crop fields and wooded areas.<ref name= "herbarium">Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: March 2019. Collectors: M. A., Preston Adams, Loran C. Anderson, C. F. Baker, Fred A. Barkley, Tom Barnes, K. E. Blum, K. A. Bradley, L. J. Brass, Michael B. Brooks, Charles T Bryson, D. Burch, - Chrysler, Andre F. Clewell, D. S. Correll, V. L. Cory, G. Crosby, Mr. H. A Davis, Mrs. H. A. Davis, Delzie Demaree, F. S. Earle, Donna Marie Eggers, Joseph Ewan, S. J. Ewer, William B. Fox, Angus Gholson, G. Gil, L. M. Gil, J. P. Gillespie, William T. Gillis, Robert K. Godfrey, H. E. Grelen, Bruce Hansen, JoAnn Hansen, Randy Haynes, Norlan C. Henderson, Brenda Herring, Don Herring, R. D. Houk, C. Jackson, S. B. Jones, Samuel B. Jones, Jr., Nancy E. Jordan, - Keenan, Annette Kessler, Gary R. Knight, R. Komarek, - Kral, Robert Kral, O. Lakela, Robert L. Lazor, S. W. Leonard, S. J. Lombardo, C. L. Lundell, Karen MacClendon, Travis MacClendon, Sidney McDaniel, Geo M. Merrill, Richard S. Mitchell, Brunelle Moon, John B. Nelson, R. A. Norris, Jackie Patman, Gwynn W. Ramsey, James D. Ray, Jr., P. L. Redfearn, P. L. Redfearn, Jr., H. R. Reed, L. L. Reese, W. D. Reese, Grady W. Reinert, H. F L. Rock, Reginald Rose-Innes, Robert Runyon, J. C. Schaffner, P. O. Schallert, A. B. Seymour, Lloyd H. Shinners, B. Shrinuk, Cecil R Slaughter, R. R. Smith, Edward S. Steele, Donald E. Stone, H. Larry E. Stripling, John W. Thieret, B. L. Turner, E. Tyson, B. M. Waddle, G. W. Waldorf, D. B. Ward, R. L. Wilbur, and – Windler. States and Counties: Florida: Alachua, Bay, Brevard, Calhoun, Collier, Columbia, Dade, Dixie, Flagler, Franklin, Gadsden, Hernando, Highlands, Hillsborough, Holmes, Indian River, Jackson, Lafayette, Lake, Lee, Leon, Levy, Liberty, Madison, Monroe, Okaloosa, Osceola, Palm Beach, Polk, Santa Rosa, Sarasota, Seminole, St Johns, St Lucie, Suwannee, Taylor, Volusia, Wakulla, Walton, and Washington. Georgia: Grady, Lanier, McIntosh, and Thomas. Alabama: Baldwin, Cleburne, Dale, Lee, and Russell. Arkansas: Ashley, Bradley, Clark, Conway, Garland, Lee, Logan, Prairie, Pulaski, and Yell. Dist of Columbia. Illinois: Jasper. Indiana: Cass. Iowa: Boone and Harrison. Louisiana: Lafayette, Rapides, and St Mary. Maryland: Baltimore. Mississippi: Forrest, Harrison, Howell, Humphreys, Jackson, Lamar, Lawrence, Leake, Leflore, Pearl River, Pocahontas, Scott, Tishomingo, and Union. Missouri: Benton, Buchanan, Harrison, Lafayette, Nodaway, St Francois, and Wayne. New Jersey: Burlington and Cape May. North Carolina: Bladen, Columbus, Currituck, Dare, Davidson, Graham, Granville, Hyde, Jackson, Rockingham, Scotland, Surry, and Swain. South Carolina: Charleston, Cherokee, Colleton, and Fairfield. Tennessee: Anderson, Montgomery, Sumner, and Williamson. Texas: Anderson, Aransas, Callahan, Cameron, Cooke, Dallas, Freestone, Hemphill, Nueces, Parker, Robertson, and Van Zandt. Virginia: Greensville, Prince Edward, and Queen Annes.</ref> | ||
| + | |||
| + | ''C. fasciculata'' showed variable changes in frequency and density in response to soil disturbance by roller chopping in Northwest Florida sandhills. In reestablished native sandhill habitat, it has shown mixed regrowth response.<ref>Hebb, E.A. (1971). Site Preparation Decreases Game Food Plants in Florida Sandhills. The Journal of Wildlife Management 35(1):155-162.</ref> It increased its frequency and biomass in response to soil disturbance by clearcutting and chopping in North Florida flatwoods forests. It has shown additional regrowth in reestablished flatwoods that were disturbed by clearcutting and chopping.<ref>Moore, W.H., B.F. Swindel, and W.S. Terry. (1982). Vegetative Response to Clearcutting and Chopping in a North Florida Flatwoods Forest. Journal of Range Management 35(2):214-218.</ref> Additionally, it was found to be an increaser in its short-term response to single mechanical soil disturbances, but neutral in its long-term response following cessation of repeated soil disturbance.<ref name=Dixon>Dixon, C. M., K. M. Robertson, A. M. Reid and M. T. Rother. 2024. Mechanical soil disturbance in a pine savanna has multiyear effects on plant species composition. Ecosphere 15(2):e4759.</ref> | ||
| + | |||
| + | Associated species: ''[[Pinus palustris]]'', ''[[Pinus elliottii]]'', ''Pinus clausa'', ''[[Quercus laevis]]'', ''[[Quercus geminata]]'', ''[[Quercus laurifolia]]'', ''Quercus margaretta'', ''[[Monarda punctata]]'', ''[[Vaccinium arboreum]]'', ''Cyrilla racemiflora'', ''[[Serenoa repens]]'', ''Chamaecrista'' sp., ''Diodia'' sp., ''Polgala'' sp., ''Aristida'' sp., ''Elymus'' sp., ''[[Scleria oligantha]]'', ''Lygodium japonicum'', and ''Chasmanthium sessiflorum''.<ref name= "herbarium"/> | ||
| + | |||
===Phenology=== <!--Timing off flowering, fruiting, seed dispersal, and environmental triggers. Cite PanFlora website if appropriate: http://www.gilnelson.com/PanFlora/ --> | ===Phenology=== <!--Timing off flowering, fruiting, seed dispersal, and environmental triggers. Cite PanFlora website if appropriate: http://www.gilnelson.com/PanFlora/ --> | ||
| + | ''C. fasciculata'' has been observed flowering between April and September with peak inflorescence in June and August.<ref>Nelson, G. [http://www.gilnelson.com/ PanFlora]: Plant data for the eastern United States with emphasis on the Southeastern Coastal Plains, Florida, and the Florida Panhandle. www.gilnelson.com/PanFlora/ Accessed: 7 DEC 2016</ref> Another source also observed ''C. fasciculata'' to flower in October, and fruit in February and April through October.<ref name= "herbarium"/> | ||
| + | |||
===Seed dispersal=== | ===Seed dispersal=== | ||
| + | This species is thought to be dispersed by consumption by vertebrates. <ref>Kirkman, L. Katherine. Unpublished database of seed dispersal mode of plants found in Coastal Plain longleaf pine-grasslands of the Jones Ecological Research Center, Georgia.</ref> | ||
| + | |||
===Seed bank and germination=== | ===Seed bank and germination=== | ||
| + | For propagation, seeds can be cold moist stratified for 56 days to improve germination success. Optimum soil temperature for germination is between 20 and 30 degrees Celsius. With this regiment, about 70% of seeds will germinate between 7 and 25 days.<ref name= "Houck"/> | ||
| + | |||
===Fire ecology=== <!--Fire tolerance, fire dependence, adaptive fire responses--> | ===Fire ecology=== <!--Fire tolerance, fire dependence, adaptive fire responses--> | ||
| + | Fire helps proliferate this species.<ref name= "Houck"/> Winter burns, rather than spring or summer burns, significantly increase ''C. fasciculata'' in frequency as well as overall biomass of the species.<ref name= "Kush">Kush, J. S., et al. (2000). Understory plant community response to season of burn in natural longleaf pine forests. Proceedings 21st Tall Timbers Fire Ecology Conference. Fire and forest ecology: innovative silviculture & vegetation management, Tallahassee, FL, Tall Timbers Research, Inc.</ref> | ||
| + | |||
===Pollination=== | ===Pollination=== | ||
| − | The following Hymenoptera families and species were observed visiting flowers of ''Chamaecrista fasciculata'' at Archbold Biological Station (Deyrup 2015): | + | The following Hymenoptera families and species were observed visiting flowers of ''Chamaecrista fasciculata'' at Archbold Biological Station: ''Apis mellifera, Bombus impatiens'' (family Apidae), ''Stenodynerus histrionalis rufustus'' (family Vespidae), members of the family Halictidae such as ''Augochlora pura, Augochloropsis metallica, A. sumptuosa, Lasioglossum coreopsis'', and ''L. placidensis'', as well as members of the family Megachilidae such as ''Coelioxys sayi, Megachile mendica'', and ''M. texana''. <ref>Deyrup, M.A. 2015. Database of observations of Hymenoptera visitations to flowers of plants on Archbold Biological Station, Florida, USA.</ref> Other Hymenoptera observed pollinating ''C. fasciculata'' include ''Dialictus coreopsis'', ''D. miniatulus'', ''D. placidensis'', ''Megachile brevis pseudobrevis'', and ''Xylocopa micans''.<ref name= "Deyrup 2002">Deyrup, M. J. E., and Beth Norden (2002). "The diversity and floral hosts of bees at the Archbold Biological Station, Florida (Hymenoptera: Apoidea)." Insecta mundi 16(1-3).</ref> Fly species in the families Syrphidae and Tachinidae (Diptera) were collected from the plant and are possible pollinators.<ref name= "Tooker">Tooker, J. F., et al. (2006). "Floral host plants of Syrphidae and Tachinidae (Diptera) of central Illinois." Annals of the Entomological Society of America 99(1): 96-112.</ref> Additionally, ''C. fasciculata'' was observed to host ground-nesting bees such as ''Pseudopanurgus albitarsis'' (family Andrenidae), bees from the family Apidae such as ''Bombus griseocollis'', ''B. impatiens'', ''B. pensylvanicus,'' and ''Melissodes bimaculatus'', as well as sweat bees from the family Halictidae such as ''Augochloropsis metallica'', ''Lasioglossum anomalum'', ''L. hitchensi'', ''L illinoense'', and ''Nomia nortoni''.<ref>Discoverlife.org [https://www.discoverlife.org/20/q?search=Bidens+albaDiscoverlife.org|Discoverlife.org]</ref> ''C. fasciculata'' also hosts aphids such as ''Aphis sp.'' (family Aphididae), leafhoppers from the family Cicadellidae such as ''Agallia sp.'', ''Empoasca sp.'' and ''Graminella nigrifrons'', and spittle bugs such as ''Clastoptera sp.'' (family Clastopteridae), true bugs such as ''Phlegyas sp.'' (family Lygaeidae) and ''Paromius longulus'' (family Rhyparochromidae), treehoppers such as ''Spissistilus festinus'' (family Membracidae), plant bugs from the family Miridae such as ''Lygus lineolaris'', ''Pseudatomoscelis seriatus'' and ''Spanagonicus albofasciatus''.<ref>Discoverlife.org [https://www.discoverlife.org/20/q?search=Bidens+albaDiscoverlife.org|Discoverlife.org]</ref> |
| − | + | ===Herbivory and toxicology=== | |
| + | ''C. fasciculata'' has raised glands on its petioles that excrete a nectar that attracts predatory ants, with the presumed adaptive benefit of encouraging ants to prey on herbivores.<ref name = boecklen>Boecklen, W.J. 1984. The role of extrafloral nectaries in the herbivore defence of Cassia fasciculata. Ecological Entomology 9:243-249.</ref> The glands have also been observed to attract bees and wasps, presumably with the same benefit to the plant.<ref>David McElveen and Kevin Robertson observation on Tall Timbers Research Station, Tallahassee, Florida, July 17 and 20, 2018.</ref> As a whole, it is approximately 5-10% of the diet for large mammals, and 10-25% of the diet for terrestrial birds where it is found.<ref name= "Miller">Miller, J.H., and K.V. Miller. 1999. Forest plants of the southeast and their wildlife uses. Southern Weed Science Society.</ref> It is a major food item for the northern bobwhite quail and other quails due to its persistence through the winter and early spring. Partridge pea is also eaten by ring-necked pheasant, greater and lesser prairie-chicken, grassland birds, mallard, field mice, and deer; it can be poisonous for livestock and is considered potentially dangerous for cattle. Upland game birds and small non-game birds, small mammals, and waterfowl utilize the litter and plant stocks of the species for cover. As well, the common sulfur butterfly (''Colias philodice'') lays eggs on the leaves so that the larvae use them as their first food source.<ref name= "Houck"/> It is also a larval host for other members of Lepidoptera, including the cloudless giant sulpher (''Phoebis sennae''), the orange sulphur (''Colias eurytheme''), and the sleepy orange (''Abaeis nicippe'').<ref name= "lady bird"/> | ||
| + | <!--===Diseases and parasites===--> | ||
| − | + | ==Conservation, cultivation, and restoration== | |
| + | For management, stands that are already established are recommended to be disked lightly in springtime to show mineral soil that the seeds can germinate on. This species can decrease in frequency if there is not regular maintenance, so light disking is necessary to remove old sod, small brush, and other weeds. Fire is also a great management tool to help proliferate this species; for best results, prescribed fire should be conducted in the winter. Mowing can also help control weeds through mowing over the top of ''C. fasciculata'' individuals.<ref name= "Houck"/> | ||
| + | To control erosion, ''C. fasciculata'' can be planted along road banks or stream banks for restoration as well as improving soil fertility. This is due to it being able to rapidly establish, fix nitrogen, to reseed, and decrease in frequency as other species start dominating the site. It is commonly included in seed mix with other grasses and forbs that are planted along roadsides to prevent establishment of weeds. Cultivars of ''C. fasciculata'' include 'Comanche' from the Knox City Plant Materials Center in Texas and 'Riley' from the Manhattan Plant Materials Center in Kansas.<ref name= "Houck"/> | ||
| − | + | ==Cultural use== | |
| + | Historically, the Cherokee Native American tribe used the species as a medicinal drug to keep ball players from tiring as well as for spells of fainting; the Seminole tribe used ''C. fasciculata'' medicinally as a drug for nausea, and used the plant as a bed for ripening persimmons.<ref name= "Houck">Houck, M. J. and J. M. Row. (2006). Plant Guide: Partridge Pea ''Chamaecrista fasciculata''. N.R.C.S. United States Department of Agriculture. Alexandria, LA.</ref> | ||
| − | + | ==Photo Gallery== | |
| + | <gallery widths=180px> | ||
| + | </gallery> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==References and notes== | ==References and notes== | ||
| − | |||
Latest revision as of 13:57, 1 August 2024
| Chamaecrista fasciculata | |
|---|---|

| |
| Photo taken by Michelle M. Smith | |
| Scientific classification | |
| Kingdom: | Plantae |
| Division: | Magnoliophyta - Flowering plants |
| Class: | Magnoliopsida - Dicotyledons |
| Order: | Fabales |
| Family: | Fabaceae ⁄ Leguminosae |
| Genus: | Chamaecrista |
| Species: | C. fasciculata |
| Binomial name | |
| Chamaecrista fasciculata (Michx.) Greene | |
| |
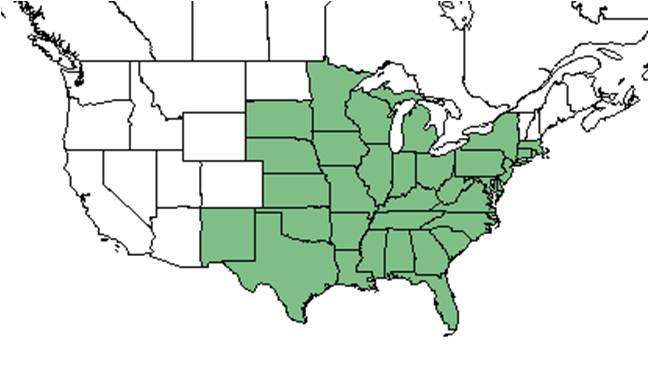
| Natural range of Chamaecrista fasciculata from USDA NRCS Plants Database. | |
Common name: Partridge Pea, common partridge-pea, tidal-marsh partridge pea
Contents
Taxonomic notes
Synonyms: Chamaecrista puberula Greene; Cassia fasciculata var. brachiata (Pollard) Pullen ex Isely; Chamaecrista brachiata Pollard; Cassia fasciculata var. macrosperma Fernald[1]
Varieties: Chamaecrista fasciculata (Michaux) Greene var. 1; Chamaecrista fasciculata (Michaux) Greene var. brachiata (Pollard) Isely; Chamaecrista fasciculata (Michaux) Greene var. fasciculata; Chamaecrista fasciculata (Michaux) Greene var. macrosperma (Fernald) C.F. Reed; Chamaecrista littoralis Pollard; Chamaecrista mississippiensis (Pollard) Pollard ex Heller; Cassia fasciculata var. littoralis (Pollard) J.F. Macbride; Cassia fasciculata var. robusta (Pollard) J.F. Macbride; Chamaecrista robusta Pollard[1]
Description
Chameacrista fasciculata is an annual herb, growing 1.5-6 dm tall from the taproot. The stems and branches are glabrous to more commonly densely puberulent with incurved trichomes and occasionally also with villous trichomes to 2 mm long. The leaves are sensitive with a sessile, depressed, saucer-shaped gland, 0.5-1.5 mm in diam. near the middle of the petiole. Leaflets 12-36, linear-oblong, 1-2.5 cm long, 2-6 mm wide, inequilateral; stipules persistent, striate. Inflorescence are 1-6 flowered axillary fascicle. Pedicels grow up to 1-2 cm long. Sepals are lanceolate in shape, growing 9-12 mm long, and are acute. Petals are bright yellow in color, almost equal, growing 1-2 cm long; stamens 10, unequal, growing 10-13 mm long. The legume are elastically dehiscent, growing 3-7 cm long, and 5-7 mm broad, and are glabrate or appressed-puberulent to villous.[2]
Chameacrista fasciculata does not have specialized underground storage units apart from its taproot.[3] Diaz-Toribio and Putz (2021) recorded this species to have an non-structural carbohydrate concentration of 76.2 mg/g (ranking 59 out of 100 species studied) and water content of 44% (ranking 35 out of 100 species studied).[3]
According to Diaz-Torbio and Putz (2021), Chamaecrista fasiculata has taproots with a below-ground to above-ground biomass ratio of 0.14 and nonstructural carbohydrate concentration of 76.2 mg g-1.[4]
Distribution
C. fasciculata is native to the eastern United States, excluding Vermont, New Hampshire, and Maine, west to New Mexico, South Dakota, and Minnesota.[5]
Ecology
Like other species in the Fabaceae family, C. fasciculata is a nitrogen-fixing plant that depends on microorganisms to help produce nitrogen compounds necessary for the plant's survival. This is conducted through a symbiosis of microorganisms inhabiting root nodules of the plant to give the plant direct access and the microorganisms a safe habitat.[6]
Habitat
C. fasciculata is a facultative upland species[5] and can be found in sandy savannas of the Gulf Coastal Plain, and bluffs, prairies, river bottoms and banks, and upland woods of the Great Plains region. Generally, this species prefers well-drained and moderately lime souls such as sandy to sandy loam soils, but the wide range of soils include slightly acidic to moderately alkaline soils.[7][8] C. fasciculata is also a common colonizer of disturbed areas[7] can be found growing on recently abandoned gopher tortoise (Gopherus polyphemus) mounds,[9], dry sand, roadsides and other disturbed areas, sand ridges, sand dunes, upland old fields, scrub clearings, pine flatwoods, sandhills, wooded ravines, salt marshes, prairies, and edges of crop fields and wooded areas.[10]
C. fasciculata showed variable changes in frequency and density in response to soil disturbance by roller chopping in Northwest Florida sandhills. In reestablished native sandhill habitat, it has shown mixed regrowth response.[11] It increased its frequency and biomass in response to soil disturbance by clearcutting and chopping in North Florida flatwoods forests. It has shown additional regrowth in reestablished flatwoods that were disturbed by clearcutting and chopping.[12] Additionally, it was found to be an increaser in its short-term response to single mechanical soil disturbances, but neutral in its long-term response following cessation of repeated soil disturbance.[13]
Associated species: Pinus palustris, Pinus elliottii, Pinus clausa, Quercus laevis, Quercus geminata, Quercus laurifolia, Quercus margaretta, Monarda punctata, Vaccinium arboreum, Cyrilla racemiflora, Serenoa repens, Chamaecrista sp., Diodia sp., Polgala sp., Aristida sp., Elymus sp., Scleria oligantha, Lygodium japonicum, and Chasmanthium sessiflorum.[10]
Phenology
C. fasciculata has been observed flowering between April and September with peak inflorescence in June and August.[14] Another source also observed C. fasciculata to flower in October, and fruit in February and April through October.[10]
Seed dispersal
This species is thought to be dispersed by consumption by vertebrates. [15]
Seed bank and germination
For propagation, seeds can be cold moist stratified for 56 days to improve germination success. Optimum soil temperature for germination is between 20 and 30 degrees Celsius. With this regiment, about 70% of seeds will germinate between 7 and 25 days.[7]
Fire ecology
Fire helps proliferate this species.[7] Winter burns, rather than spring or summer burns, significantly increase C. fasciculata in frequency as well as overall biomass of the species.[16]
Pollination
The following Hymenoptera families and species were observed visiting flowers of Chamaecrista fasciculata at Archbold Biological Station: Apis mellifera, Bombus impatiens (family Apidae), Stenodynerus histrionalis rufustus (family Vespidae), members of the family Halictidae such as Augochlora pura, Augochloropsis metallica, A. sumptuosa, Lasioglossum coreopsis, and L. placidensis, as well as members of the family Megachilidae such as Coelioxys sayi, Megachile mendica, and M. texana. [17] Other Hymenoptera observed pollinating C. fasciculata include Dialictus coreopsis, D. miniatulus, D. placidensis, Megachile brevis pseudobrevis, and Xylocopa micans.[18] Fly species in the families Syrphidae and Tachinidae (Diptera) were collected from the plant and are possible pollinators.[19] Additionally, C. fasciculata was observed to host ground-nesting bees such as Pseudopanurgus albitarsis (family Andrenidae), bees from the family Apidae such as Bombus griseocollis, B. impatiens, B. pensylvanicus, and Melissodes bimaculatus, as well as sweat bees from the family Halictidae such as Augochloropsis metallica, Lasioglossum anomalum, L. hitchensi, L illinoense, and Nomia nortoni.[20] C. fasciculata also hosts aphids such as Aphis sp. (family Aphididae), leafhoppers from the family Cicadellidae such as Agallia sp., Empoasca sp. and Graminella nigrifrons, and spittle bugs such as Clastoptera sp. (family Clastopteridae), true bugs such as Phlegyas sp. (family Lygaeidae) and Paromius longulus (family Rhyparochromidae), treehoppers such as Spissistilus festinus (family Membracidae), plant bugs from the family Miridae such as Lygus lineolaris, Pseudatomoscelis seriatus and Spanagonicus albofasciatus.[21]
Herbivory and toxicology
C. fasciculata has raised glands on its petioles that excrete a nectar that attracts predatory ants, with the presumed adaptive benefit of encouraging ants to prey on herbivores.[22] The glands have also been observed to attract bees and wasps, presumably with the same benefit to the plant.[23] As a whole, it is approximately 5-10% of the diet for large mammals, and 10-25% of the diet for terrestrial birds where it is found.[24] It is a major food item for the northern bobwhite quail and other quails due to its persistence through the winter and early spring. Partridge pea is also eaten by ring-necked pheasant, greater and lesser prairie-chicken, grassland birds, mallard, field mice, and deer; it can be poisonous for livestock and is considered potentially dangerous for cattle. Upland game birds and small non-game birds, small mammals, and waterfowl utilize the litter and plant stocks of the species for cover. As well, the common sulfur butterfly (Colias philodice) lays eggs on the leaves so that the larvae use them as their first food source.[7] It is also a larval host for other members of Lepidoptera, including the cloudless giant sulpher (Phoebis sennae), the orange sulphur (Colias eurytheme), and the sleepy orange (Abaeis nicippe).[6]
Conservation, cultivation, and restoration
For management, stands that are already established are recommended to be disked lightly in springtime to show mineral soil that the seeds can germinate on. This species can decrease in frequency if there is not regular maintenance, so light disking is necessary to remove old sod, small brush, and other weeds. Fire is also a great management tool to help proliferate this species; for best results, prescribed fire should be conducted in the winter. Mowing can also help control weeds through mowing over the top of C. fasciculata individuals.[7] To control erosion, C. fasciculata can be planted along road banks or stream banks for restoration as well as improving soil fertility. This is due to it being able to rapidly establish, fix nitrogen, to reseed, and decrease in frequency as other species start dominating the site. It is commonly included in seed mix with other grasses and forbs that are planted along roadsides to prevent establishment of weeds. Cultivars of C. fasciculata include 'Comanche' from the Knox City Plant Materials Center in Texas and 'Riley' from the Manhattan Plant Materials Center in Kansas.[7]
Cultural use
Historically, the Cherokee Native American tribe used the species as a medicinal drug to keep ball players from tiring as well as for spells of fainting; the Seminole tribe used C. fasciculata medicinally as a drug for nausea, and used the plant as a bed for ripening persimmons.[7]
Photo Gallery
References and notes
- ↑ 1.0 1.1 Weakley, A.S. 2020. Flora of the Southeastern United States. Edition of 20 October 2020. University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
- ↑ Radford, Albert E., Harry E. Ahles, and C. Ritchie Bell. Manual of the Vascular Flora of the Carolinas. 1964, 1968. The University of North Carolina Press. 577-8. Print.
- ↑ 3.0 3.1 Diaz-Toribio, M.H. and F. E. Putz 2021. Underground carbohydrate stores and storage organs in fire-maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108: 432-442.
- ↑ Diaz‐Toribio, M. H. and F. E. Putz. 2021. Underground carbohydrate stores and storage organs in fire‐maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108(3):432-442.
- ↑ 5.0 5.1 USDA, NRCS. (2016). The PLANTS Database (http://plants.usda.gov, 4 April 2019). National Plant Data Team, Greensboro, NC 27401-4901 USA.
- ↑ 6.0 6.1 [[1]] Lady Bird Johnson Wildflower Center. Accessed: April 4, 2019
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 Houck, M. J. and J. M. Row. (2006). Plant Guide: Partridge Pea Chamaecrista fasciculata. N.R.C.S. United States Department of Agriculture. Alexandria, LA.
- ↑ USDA NRCS Plant Materials Program and J. M. Row. (2006). Plant Fact Sheet: Showy Partridge Pea Chamaecrista fasciculata.N.R.C.S. United States Department of Agriculture. Manhattan, KS.
- ↑ Kaczor, S. A. and D. C. Hartnett (1990). "Gopher tortoise (Gopherus polyphemus) effects on soils and vegetation in a Florida sandhill." American Midland Naturalist 123: 100-111.
- ↑ 10.0 10.1 10.2 Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: March 2019. Collectors: M. A., Preston Adams, Loran C. Anderson, C. F. Baker, Fred A. Barkley, Tom Barnes, K. E. Blum, K. A. Bradley, L. J. Brass, Michael B. Brooks, Charles T Bryson, D. Burch, - Chrysler, Andre F. Clewell, D. S. Correll, V. L. Cory, G. Crosby, Mr. H. A Davis, Mrs. H. A. Davis, Delzie Demaree, F. S. Earle, Donna Marie Eggers, Joseph Ewan, S. J. Ewer, William B. Fox, Angus Gholson, G. Gil, L. M. Gil, J. P. Gillespie, William T. Gillis, Robert K. Godfrey, H. E. Grelen, Bruce Hansen, JoAnn Hansen, Randy Haynes, Norlan C. Henderson, Brenda Herring, Don Herring, R. D. Houk, C. Jackson, S. B. Jones, Samuel B. Jones, Jr., Nancy E. Jordan, - Keenan, Annette Kessler, Gary R. Knight, R. Komarek, - Kral, Robert Kral, O. Lakela, Robert L. Lazor, S. W. Leonard, S. J. Lombardo, C. L. Lundell, Karen MacClendon, Travis MacClendon, Sidney McDaniel, Geo M. Merrill, Richard S. Mitchell, Brunelle Moon, John B. Nelson, R. A. Norris, Jackie Patman, Gwynn W. Ramsey, James D. Ray, Jr., P. L. Redfearn, P. L. Redfearn, Jr., H. R. Reed, L. L. Reese, W. D. Reese, Grady W. Reinert, H. F L. Rock, Reginald Rose-Innes, Robert Runyon, J. C. Schaffner, P. O. Schallert, A. B. Seymour, Lloyd H. Shinners, B. Shrinuk, Cecil R Slaughter, R. R. Smith, Edward S. Steele, Donald E. Stone, H. Larry E. Stripling, John W. Thieret, B. L. Turner, E. Tyson, B. M. Waddle, G. W. Waldorf, D. B. Ward, R. L. Wilbur, and – Windler. States and Counties: Florida: Alachua, Bay, Brevard, Calhoun, Collier, Columbia, Dade, Dixie, Flagler, Franklin, Gadsden, Hernando, Highlands, Hillsborough, Holmes, Indian River, Jackson, Lafayette, Lake, Lee, Leon, Levy, Liberty, Madison, Monroe, Okaloosa, Osceola, Palm Beach, Polk, Santa Rosa, Sarasota, Seminole, St Johns, St Lucie, Suwannee, Taylor, Volusia, Wakulla, Walton, and Washington. Georgia: Grady, Lanier, McIntosh, and Thomas. Alabama: Baldwin, Cleburne, Dale, Lee, and Russell. Arkansas: Ashley, Bradley, Clark, Conway, Garland, Lee, Logan, Prairie, Pulaski, and Yell. Dist of Columbia. Illinois: Jasper. Indiana: Cass. Iowa: Boone and Harrison. Louisiana: Lafayette, Rapides, and St Mary. Maryland: Baltimore. Mississippi: Forrest, Harrison, Howell, Humphreys, Jackson, Lamar, Lawrence, Leake, Leflore, Pearl River, Pocahontas, Scott, Tishomingo, and Union. Missouri: Benton, Buchanan, Harrison, Lafayette, Nodaway, St Francois, and Wayne. New Jersey: Burlington and Cape May. North Carolina: Bladen, Columbus, Currituck, Dare, Davidson, Graham, Granville, Hyde, Jackson, Rockingham, Scotland, Surry, and Swain. South Carolina: Charleston, Cherokee, Colleton, and Fairfield. Tennessee: Anderson, Montgomery, Sumner, and Williamson. Texas: Anderson, Aransas, Callahan, Cameron, Cooke, Dallas, Freestone, Hemphill, Nueces, Parker, Robertson, and Van Zandt. Virginia: Greensville, Prince Edward, and Queen Annes.
- ↑ Hebb, E.A. (1971). Site Preparation Decreases Game Food Plants in Florida Sandhills. The Journal of Wildlife Management 35(1):155-162.
- ↑ Moore, W.H., B.F. Swindel, and W.S. Terry. (1982). Vegetative Response to Clearcutting and Chopping in a North Florida Flatwoods Forest. Journal of Range Management 35(2):214-218.
- ↑ Dixon, C. M., K. M. Robertson, A. M. Reid and M. T. Rother. 2024. Mechanical soil disturbance in a pine savanna has multiyear effects on plant species composition. Ecosphere 15(2):e4759.
- ↑ Nelson, G. PanFlora: Plant data for the eastern United States with emphasis on the Southeastern Coastal Plains, Florida, and the Florida Panhandle. www.gilnelson.com/PanFlora/ Accessed: 7 DEC 2016
- ↑ Kirkman, L. Katherine. Unpublished database of seed dispersal mode of plants found in Coastal Plain longleaf pine-grasslands of the Jones Ecological Research Center, Georgia.
- ↑ Kush, J. S., et al. (2000). Understory plant community response to season of burn in natural longleaf pine forests. Proceedings 21st Tall Timbers Fire Ecology Conference. Fire and forest ecology: innovative silviculture & vegetation management, Tallahassee, FL, Tall Timbers Research, Inc.
- ↑ Deyrup, M.A. 2015. Database of observations of Hymenoptera visitations to flowers of plants on Archbold Biological Station, Florida, USA.
- ↑ Deyrup, M. J. E., and Beth Norden (2002). "The diversity and floral hosts of bees at the Archbold Biological Station, Florida (Hymenoptera: Apoidea)." Insecta mundi 16(1-3).
- ↑ Tooker, J. F., et al. (2006). "Floral host plants of Syrphidae and Tachinidae (Diptera) of central Illinois." Annals of the Entomological Society of America 99(1): 96-112.
- ↑ Discoverlife.org [2]
- ↑ Discoverlife.org [3]
- ↑ Boecklen, W.J. 1984. The role of extrafloral nectaries in the herbivore defence of Cassia fasciculata. Ecological Entomology 9:243-249.
- ↑ David McElveen and Kevin Robertson observation on Tall Timbers Research Station, Tallahassee, Florida, July 17 and 20, 2018.
- ↑ Miller, J.H., and K.V. Miller. 1999. Forest plants of the southeast and their wildlife uses. Southern Weed Science Society.