Callicarpa americana
| Callicarpa americana | |
|---|---|

| |
| Photo taken by Kevin Robertson (2015) | |
| Scientific classification | |
| Kingdom: | Plantae |
| Division: | Magnoliophyta - Flowering plants |
| Class: | Magnoliopsida - Dicotyledons |
| Order: | Lamiales |
| Family: | Verbenaceae |
| Genus: | Callicarpa |
| Species: | C. americana |
| Binomial name | |
| Callicarpa americana L. | |
| |
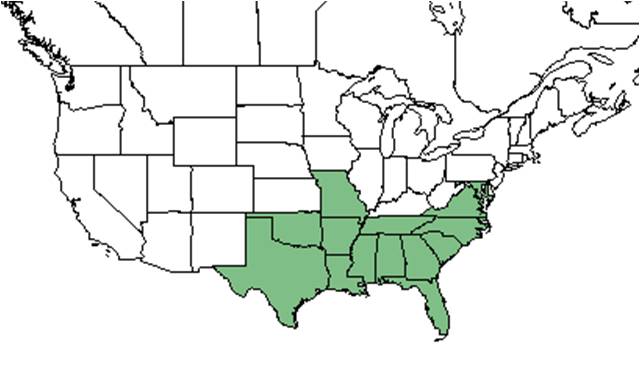
| Natural range of Callicarpa americana from USDA NRCS Plants Database. | |
Common names: Beautyberry, American Beautyberry; French-Mulberry
Contents
Taxonomic notes
In Greek, Callicarpa derives from callos meaning "beauty" and carpos meaning "fruit".[1]
Synonyms: none[2]
Varieties: none[2]
Description
Generally for the Callicarpa genus, these plants grow up to 1 - 2.5 m tall with twigs displaying stellate pubescence. The petiolate leaves are simple, opposite or subopposite, have short pubescent above, and are ovate, ovate-lanceolate, or elliptic and acute to acuminate in shape. The flowers are in axillary cymes. The calyx is shallowly 5-toothed and grows 0.5 - 2 mm long. The petals are united ca. 1/2-2/3 their length. The lobes 5, are spreading, lavender to pinkish in color and grow 3 - 5 mm long. The stamens protrude from the flower and the stigma is slightly 2-lobed. The drupe (a type of fruit) is 4-seeded, lavendar to purple or rarely white in color and are globose in shape. The seeds are light yellow to brown in color, ellipsoid to orbicular rounded on the back, and are flattened on the inner surface. [3]
Specifically, for Callicarpa americana, the leaves are ovate to ovate-lanceolate in shape, grow 7 - 15 cm wide with stellate pubescence on the underside, have crenate to serrate margins, and at the base are widely cuneate or rounded. The petioles grow 1.5 - 3.5 cm long, and are scurfy stellate like the twigs. The cymes are shorter than the subtending petioles. The peduncles grow 1 - 5 mm long. The drupe grows 3 - 5 mm long. The pyrenes grow 2.3 mm long. [3]
Callicarpa americana does not have specialized underground storage units apart from its rhizomes.[4] Diaz-Toribio and Putz (2021) recorded this species to have an non-structural carbohydrate concentration of 104.3 mg/g (ranking 50 out of 100 species studied).[4]
According to Diaz-Torbio and Putz (2021), Callicarpa americana has rhizomes with a below-ground to above-ground biomass ratio of 2.06 and nonstructural carbohydrate concentration of 104.3 mh g-1.[5]
Distribution
C. ameriana is native to the southeastern United States, ranging from Maryland to south Florida and Texas. It can also be found in Mexico and the West Indies.[6]
Ecology
Habitat
The species is associated with longleaf pine forest ecosystems.[7] C. americana has also been observed in sandy soil coastal hammocks, wooded floodplains, calcareous bluffs, margins of creek swamps and swamps, dry sandy slopes, woodland forest edges, understory shrub thickets, flat creeks, and waterfronts.[8] It is a dominant plant species on abandoned crop sites of upland pinelands.[9] One study found that C. americana was more abundant in tornado-damaged areas containing an open canopy rather than undamaged areas.[10]
C. americana did not respond to soil disturbance by clearcutting and chopping in North Florida flatwoods forests.[11] It had variable changes in frequency in response to soil disturbance by roller chopping, and it was found to decrease its frequency in response to disturbance by a KG blade in East Texas Loblolly Pine-Hardwood Forests. It has shown either additional growth or resistance to regrowth in reestablished pine forests that were disturbed by roller chopping. Additionally, it has shown resistance to regrowth in the reestablished forests that were disturbed by KG blade.[12] However, C. americana was found to be an increaser in its short-term response to single mechanical soil disturbances as well as in its long-term response following cessation of repeated soil disturbance.[13]
Associated species: Quercus sp., Ulmus sp., Ligustrum sp., Liquidambar styraciflua, Prunus sp., Carya sp., Sabal palmetto, and Sabal minor.[8]
Phenology
C. americana has been observed flowering from May to August with peak inflorescence in June.[14] Fruiting period is normally August through January.[15] The later blooms and developing fruit persist into the winter.[6]
Seed dispersal
The seeds are dispersed by animals and birds through consumption.[1][16]
Seed bank and germination
Seeds that are planted in the fall commonly germinate in the spring, but C. americana seeds can persist in the seed bank for several years.[1]
Fire ecology
Populations of Callicarpa americana have been known to persist through repeated annual burns,[17][18] and has been observed growing in sites that were previously burned within the last year.[8] The species has overall increased production in response to fire.[19] C. americana poses a low wildfire hazard to the wildland-urban interface due to its high foliar moisture content and its low fuel bed bulk density.[20] A study in Arkansas found that C. americana increases in understory cover with a 6 year or 9 year burn cycle.[21] The species has been shown to increase protein and phosphoric acid levels in response to fire and decrease levels of calcium.[22]
Pollination
The flowers of Callicarpa americana have been observed to host bees such as Apis mellifera (family Apidae), sweat bees from the family Halictidae such as Agapostemon splendens, Augochlora pura, Halictus poeyi and Lasioglossum placidensis, and leafcutting bees such as Megachile brevis pseudobrevis (family Megachilidae).[23] Other Hymenoptera that pollinate C. americana include Dialictus placidensis and Halictus ligatus (family Halictidae).[24]
Herbivory and toxicology
C. americana is a moderate portion of large mammal diets, ranging from 10-25% of their average diet, and a low portion of small mammal and terrestrial bird diets, from 5-10% of their diet.[25][26] Since the fruit is high in moisture content, it is an important source of food for over 40 species of songbirds, like the brown thrasher, purple finch, american robin, and eastern towhee. The fruit clusters are also eaten by foxes, opossum, armadillo, white tailed deer, squirrels, and raccoon.[1] The Florida marsh rabbit has been observed to browse on the plant.[27] It is also a well known food and cover plant for the northern bobwhite quail.[28] The white tailed deer also browse on the leaves when other preferred food is not available. As well, cattle browse on the twigs in the winter and twigs and leaves in the spring.
Diseases and parasites
Leaf spots (Atractilina callicarpae) and black mold (Meliola cookeana) affect plants in the Callicarpa genus.[1] It is a host plant for Brevipalpus californicus, B. obovatus, and B. phoenicis, which are all false spider mites that cause citrus leprosis.[29]
Conservation, cultivation, and restoration
It is listed as endangered and extirpated by the Maryland Department of Natural Resources.[30] For ornamental management purposes, pruning the plants in the fall or winter will maintain its form, and old stems should be cut when pruning since only new growth produces fruit.[1] While there is no official cultivars of C. americana, there is a variety available (C. americana var. lactea) that produces white fruit that is available at some nurseries.[1]
Cultural use
Historically, C. americana was utilized by Native American tribes for many medicinal purposes, including malarial fevers and rheumatism. As well, the roots were used for stomachaches, dizziness, and dysentry, and the roots and berries were used to treat colic by boiling them and drinking it.[1] The leaves were also used for dropsy, an old term for edema.[31] Farmers used the plant in the early 20th century as a mosquito repellent for horses and mules by crushing the leaves and placing them under the harnesses, and would use this same method on themselves as well.[1] Additionally, cuttings of the plant are considered an aesthetic table ornament.[32]
Photo Gallery
References and notes
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Brakie, M. 2010. Plant fact sheet for American beautyberry (Callicarpa americana). USDA-Natural Resources Conservation Service, East Texas Plant Materials Center. Nacogdoches, TX, 75964.
- ↑ 2.0 2.1 Weakley, A.S. 2020. Flora of the Southeastern United States. Edition of 20 October 2020. University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
- ↑ 3.0 3.1 Radford, Albert E., Harry E. Ahles, and C. Ritchie Bell. Manual of the Vascular Flora of the Carolinas. 1964, 1968. The University of North Carolina Press. 894. Print.
- ↑ 4.0 4.1 Diaz-Toribio, M.H. and F. E. Putz 2021. Underground carbohydrate stores and storage organs in fire-maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108: 432-442.
- ↑ Diaz-Torbio, M. H. and F. E. Putz. 2021. Underground carbohydrate stores and storage organs in fire-maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108(3):432-422.
- ↑ 6.0 6.1 Weakley, A. S. (2015). Flora of the Southern and Mid-Atlantic States. Chapel Hill, NC, University of North Carolina Herbarium.
- ↑ Brockway, D. G., et al. (2005). Restoration of longleaf pine ecosystems. F. S. United States Department of Agriculture, Southern Research Station.
- ↑ 8.0 8.1 8.2 Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: March 2019. Collectors: Harry E. Ahles, Loran C. Anderson, W. R. Anderson, W. P. Adams, Joel A. Barnes, Tom Barnes, Don Blake, H. L. Blomquist, Kurt Blum, J. Bonk, Jane Brockmann, Michael B. Brooks, Kathy Craddock Burks, Burnett, Chris Cooksey, D. S. Correll, Delzie Demaree, R. F. Doren, Patricia Elliot, Joseph Ewan, J. Ferborgh, G. Fleming, Suellen Folensbee, P. Genelle, A. Gholson, Jr., J. P. Gillespie, M. Gillespie, Robert K. Godfrey, C. J. Hansen, Bruce Hansen, JoAnn Hansen, James W. Hardin, Randy Haynes, P. Hilsenbeck, R. Hilsenbeck, Ron Hughes, Samuel B. Jones, Jr., King, Gary R. Knight, R. Komarek, R. Kral, O. Lakela, Robert L. Lazor, Sidney McDaniel, Marc Minno, Richard S. Mitchell, Florence Montgomery, R. Nims, T. Nims, R. Nunan, John C. Ogden, Parker, Laurie Pipkorn, J. Poppleton, Elmer C. Prichard, Gwynn W. Ramsey, Paul L. Redfearn, Jr., Josephine Skehan, Cecil R. Slaughter, William R. Stimson, John W. Thieret, R. E. Weaver, Jr., R. L. Wilbur, Roomie Wilson, Richard P. Wunderlin, and T. Wunderlin. States and Counties: Florida: Broward, Calhoun, Citrus, Dade, Escambia, Franklin, Gadsden, Indian River, Jackson, Jefferson, Leon, Levy, Liberty, Madison, Monroe, Osceola, Seminole, Volusia, and Wakulla. Alabama: Chambers, Houston, Limestone, Lowndes, Monroe, and Pickens. Georgia: Decatur, De Kalb, Grady, McIntosh, Oglethorpe, and Thomas. Mississippi: Forrest, Hancock, Jackson, Pearl River, and Pike. South Carolina: Dorchester, Pickens, and Richland. Louisiana: Evangeline, Iberia, St Landry, and Tangipahoa. Arkansas: Hot Spring and Stone. Texas: Bexar, Jasper, and Van Zandt. North Carolina: Carteret, Dare, Johnston, and Transylvania. Virginia: Norfolk.
- ↑ Ostertag, T. E. and K. M. Robertson (2007). A comparison of native versus old-field vegetation in upland pinelands managed with frequent fire, south Georgia, USA. Proceedings of the 23rd Tall Timbers Fire Ecology Conference: Fire in Grassland and Shrubland Ecosystems, Tallahassee, Tall Timbers Research Station.
- ↑ Brewer, S. J., et al. (2012). "Do natural disturbances or the forestry practices that follow them convert forests to early-successional communities?" Ecological Applications 22: 442-458.
- ↑ Moore, W.H., B.F. Swindel, and W.S. Terry. (1982). Vegetative Response to Clearcutting and Chopping in a North Florida Flatwoods Forest. Journal of Range Management 35(2):214-218.
- ↑ Stransky, J.J., J.C. Huntley, and Wanda J. Risner. (1986). Net Community Production Dynamics in the Herb-Shrub Stratum of a Loblolly Pine-Hardwood Forest: Effects of CLearcutting and Site Preparation. Gen. Tech. Rep. SO-61. New Orleans, LA: U.S. Dept of Agriculture, Forest Service, Southern Forest Experiment Station. 11 p.
- ↑ Dixon, C. M., K. M. Robertson, A. M. Reid and M. T. Rother. 2024. Mechanical soil disturbance in a pine savanna has multiyear effects on plant species composition. Ecosphere 15(2):e4759.
- ↑ Nelson, G. PanFlora: Plant data for the eastern United States with emphasis on the Southeastern Coastal Plains, Florida, and the Florida Panhandle. www.gilnelson.com/PanFlora/ Accessed: 7 DEC 2016
- ↑ Skeate, S. T. (1987). "Interactions between birds and fruits in a Northern Florida hammock community." Ecology 68(2): 297-309.
- ↑ Creech, M. N., et al. (2012). "Alteration and Recovery of Slash Pile Burn Sites in the Restoration of a Fire-Maintained Ecosystem." Restoration Ecology 20(4): 505-516.
- ↑ Robertson, K.M. Unpublished data collected from Pebble Hill Fire Plots, Pebble Hill Plantation, Thomasville, Georgia.
- ↑ Glitzenstein, J. S., D. R. Streng, R. E. Masters, K. M. Robertson and S. M. Hermann 2012. Fire-frequency effects on vegetation in north Florida pinelands: Another look at the long-term Stoddard Fire Research Plots at Tall Timbers Research Station. Forest Ecology and Management 264: 197-209.
- ↑ Lay, D. W. (1967). "Browse palatability and the effects of prescribed burning in southern pine forests." Journal of Forestry 65: 826-828.
- ↑ Behm, A. L., et al. (2004). "Flammability of native understory species in pine flatwood and hardwood hammock ecosystems and implications for the wildland-urban interface." International Journal of Wildland Fire 13: 355-365.
- ↑ Cain, M. D., et al. (1998). "Prescribed fire effects on structure in uneven-aged stands of loblolly and shortleaf pines." Wildlife Society Bulletin 26: 209-218.
- ↑ Lay, D. W. (1957). "Browse quality and the effects of prescribed burning in southern pine forests." Journal of Forestry 55: 342-347.
- ↑ Deyrup, M.A. 2015. Database of observations of Hymenoptera visitations to flowers of plants on Archbold Biological Station, Florida, USA.
- ↑ Deyrup, M. J. E., and Beth Norden (2002). "The diversity and floral hosts of bees at the Archbold Biological Station, Florida (Hymenoptera: Apoidea)." Insecta mundi 16(1-3).
- ↑ Miller, J.H., and K.V. Miller. 1999. Forest plants of the southeast and their wildlife uses. Southern Weed Science Society.
- ↑ Yarrow, G.K., and D.T. Yarrow. 1999. Managing wildlife. Sweet Water Press. Birmingham.
- ↑ Blair, W. F. (1936). "The Florida marsh rabbit." Journal of Mammalogy 17(3): 197-207.
- ↑ Chenault, T. P. (1940). "The phenology of some bob-white food and cover plants in Brazos County, Texas." The Journal of Wildlife Management 4(4): 359-368.
- ↑ Childers, C. C., et al. (2003). "Host plants of Brevipalpus californicus, B. obovatus, and B. phoenicis (Acari: Tenuipalpidae) and their potential involvement in the spread of viral diseases vectored by these mites." Experimental & Applied Acarology 30: 29-105.
- ↑ USDA, NRCS. (2016). The PLANTS Database (http://plants.usda.gov, 29 March 2019). National Plant Data Team, Greensboro, NC 27401-4901 USA.
- ↑ Nickell, J. M. (1911). J.M.Nickell's botanical ready reference : especially designed for druggists and physicians : containing all of the botanical drugs known up to the present time, giving their medical properties, and all of their botanical, common, pharmacopoeal and German common (in German) names. Chicago, IL, Murray & Nickell MFG. Co.
- ↑ Fernald, et al. 1958. Edible Plants of Eastern North America. Harper and Row Publishers, New York.