Difference between revisions of "Heterotheca subaxillaris"
(→Photo Gallery) |
|||
| Line 19: | Line 19: | ||
Common name: camphorweed | Common name: camphorweed | ||
| − | + | ==Taxonomic notes== | |
| + | Synonyms: ''Heterotheca subaxillaris'' (Lamarck) Britton & Rusby var. ''subaxillaris''; ''H. subaxillaris'' ssp. ''subaxillaris'' | ||
==Description== | ==Description== | ||
<!-- Basic life history facts such as annual/perrenial, monoecious/dioecious, root morphology, seed type, etc. --> | <!-- Basic life history facts such as annual/perrenial, monoecious/dioecious, root morphology, seed type, etc. --> | ||
Revision as of 12:49, 15 March 2016
| Heterotheca subaxillaris | |
|---|---|

| |
| Photo by Allen Boatman, Atlas of Florida Vascular Plants | |
| Scientific classification | |
| Kingdom: | Plantae |
| Division: | Magnoliophyta - Flowering plants |
| Class: | Magnoliopsida - Dicotyledons |
| Order: | Asterales |
| Family: | Asteraceae ⁄ Compositae |
| Genus: | Heterotheca |
| Species: | H. subaxillaris |
| Binomial name | |
| Heterotheca subaxillaris (Lam.) Britton & Rusby | |
| |
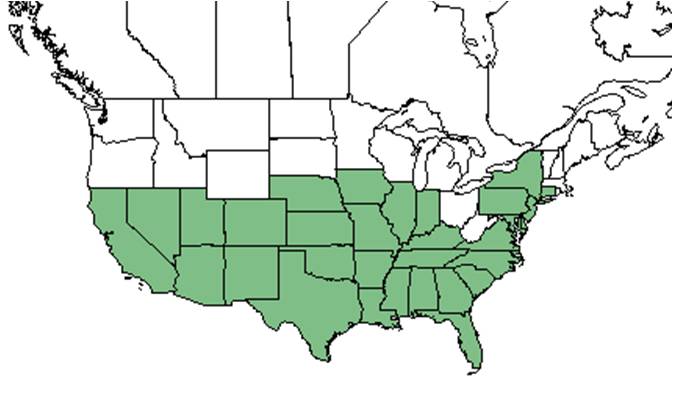
| Natural range of Heterotheca subaxillaris from USDA NRCS Plants Database. | |
Common name: camphorweed
Contents
Taxonomic notes
Synonyms: Heterotheca subaxillaris (Lamarck) Britton & Rusby var. subaxillaris; H. subaxillaris ssp. subaxillaris
Description
A description of Heterotheca subaxillaris is provided in The Flora of North America.
Heterotheca subaxillaris is an annual in temperate climates and a short lived perennial in subtropical climates (Lonard et al. 2011). Leaves are simple and are alternately arranged, with one leaf per node. The leaves produce a camphor-like aroma which defers herbivores [1]. The yellow composite flowers are arranged in diffuse, paniculate corymbs. It is a C3 plant in carbon fixation (Lonard et al. 2011).
Ecology
Habitat
In the Coastal Plain in Florida and Georgia, H. subaxillaris occurs in open sand with sea oats, pine-scrub oak sand ridges, longleaf pine-wiregrass savannas, and upper beaches. It thrives in disturbed areas and has been observed to be a ruderal species that quickly colonizes xeric habitats. It is able to compete well against other species in disturbed habitats due to the overwintering rosettes shading competing seeds (Lonard et al. 2011). It has been found in disturbed areas such as railroad bridges, powerline corridors, vacant lots, along highways, wet roadside ditches, near phosphate ponds, fire lines, disturbed coastal dunes, and cleared sand pine-evergreen oak scrub (FSU Herbarium). Areas in which it inhabits typically have low levels of soil nutrients and high sand temperature (Lonard et al. 2011) when nitrogen levels are low, it produces an increased amount of allelochemicals and allocates more carbon to root growth (Mihaliak and Lincoln 1985). Soil types include loamy sand and sand (FSU Herbarium). Associated species include Andropogon, Baccharis, Setaria, Cenchrus, Distichlis, Paspalum urvillei, P. notatum, Eragrostis oxylepis, Eleusine indica, Digitaria sanguinalis, Cyperus surinamensis, Ambrosia artemisiifolia, Strophostyles helvola, Solanum americanum, S. sisymbrifolium, Daubentonia drummondii, and Sesbania exaltata (FSU Herbarium).
Phenology
Flowers March through November and fruits in September (FSU Herbarium). It is self-incompatible and depends on insects for pollination (Lonard et al. 2011). H. subaxillaris has been observed flowering at different times in different areas, probably an adaptation to a shorter growing season (Burk 1966).
Seed dispersal
Seeds are dispersed by wind (Lonard et al. 2011).
Seed bank and germination
H. subaxillaris is a heterocarpic species (Gibson and Tomlinson 2002), the achenes produced by the ray flowers lack a pappus and require a long period of dormancy [1]. The achenes produced are dimorphic and lack albumin (Lonard et al. 2011).
Germination occurs in the spring or fall; with seeds that germinate in the fall overwintering in a rosette and forming a taproot (Guertin and Halvorson 2003). In coastal dunes, there is an absence of a persistent seed bank for H. subaxillaris; however, it has been observed to germinate from a highly disturbed seed bank. Seedlings form dense mats below the dead parent plants in late winter and early spring (Lonard et al. 2011)
Pollination
The following Hymenoptera families and species were observed visiting flowers of Heterotheca subaxillaris at Archbold Biological Station (Deyrup 2015):
Andrenidae: Andrena fulvipennis
Halictidae: Agapostemon splendens, Augochloropsis metallica, A. sumptuosa, Halictus poeyi, Lasioglossum nymphalis, L. tamiamensis
Megachilidae: Anthidium maculifrons, Coelioxys mexicana, Megachile albitarsis, M. brevis pseudobrevis, M. inimica, M. xylocopoides
Sphecidae: Bicyrtes capnoptera, Microbembex monodonta, Tachytes validus
Vespidae: Pachodynerus erynnis, Stenodynerus fundatiformis, Zethus spinipes
Use by animals
In defoliated plants, there is an increased biomass allocation to shoot growth, maintaining a balance between carbon and nutrient income similar to non defoliated plants. Defoliated plants have a slow growth rate, suggesting that even though proportionally greater allocation to the shoots occurs after defoliation, both carbon income and nutrient uptake are reduced (Mihaliak and Lincoln 1989).
Photo Gallery
References and notes
Burk, C. John. “Rainfall Periodicity as a Major Factor in the Formation of Flowering Races of Camphorweed (heterotheca Subaxillaris)”. American Journal of Botany 53.9 (1966): 933–936.
Charles A. Mihaliak, and David E. Lincoln. “Plant Biomass Partitioning and Chemical Defense: Response to Defoliation and Nitrate Limitation”. Oecologia 80.1 (1989): 122–126.
Cheplick, G.P., 2005. Patterns in the distribution of American beachgrass (Ammophila breviligulata) and the density and reproduction of annual plants on a coastal beach. Plant Ecology, 180, 57-67
Deyrup, M.A. and N.D. 2015. Database of observations of Hymenoptera visitations to flowers of plants on Archbold Biological Station, Florida, USA.
Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: October 2015. Collectors: Loran C. Anderson, Robert Blaisdell, A.F. Clewell, G. Crosby, M. Darst, Dawn Doran, Geriuld Wilhelm, Mark A. Garland, Robert K. Godfrey, Bruce Hansen, JoAnn Hansen, C. Jackson, R. Kral, Robert L. Lazor, Robert J. Lemaire, D.W. Mather, Travis MacClendon, Sidney McDaniel, Richard S. Mitchell, John B. Nelson, Gwynn W. Ramsey, R.L. Redfearn Jr., Cecil R. Slaughter, H. Larry Stripling, Bian Tan, D.B. Ward. States and Counties: Florida: Bay, Calhoun, Citrus, Clay, Columbia, Escambia, Franklin, Hernando, Indian River, Jackson, Leon, Levy, Madison, Marion, Okaloosa, Pinellas, Polk, Suwannee, St. Lucie, Wakulla. Georgia: Jasper. Compiled by Tall Timbers Research Station and Land Conservancy.
Guertin, P. and Halvorson, W.L. 2003. Factsheet for: Heterotheca subaxillaris (Lam. )Brtit & Rusby. Tucson, Arizona: U.S. Geological Survey 25 p.
J. Phil Gibson and Amelia D. Tomlinson (2002). "Genetic Diversity and Mating System Comparisons between Ray and Disc Achene Seed Pools of the Heterocarpic Species Heterotheca subaxillaris (Asteraceae)." International Journal of Plant Sciences 163(6): 1025-1034
Lonard, Robert I., Frank W. Judd, and Richard Stalter. “Biological Flora of Coastal Dunes and Wetlands: Heterotheca Subaxillaris (J. De Lamarck) N. Britton & H. Rusby”. Journal of Coastal Research 27.6 (2011): 1052–1058.
Olsen, Kenneth M.. “Pollination Effectiveness and Pollinator Importance in a Population of Heterotheca Subaxillaris (asteraceae)”. Oecologia 109.1 (1997): 114–121