Galactia regularis
| Galactia regularis | |
|---|---|

| |
| Photo was taken by Gil Nelson | |
| Scientific classification | |
| Kingdom: | Plantae |
| Division: | Magnoliophyta - Flowering plants |
| Class: | Magnoliopsida – Dicotyledons |
| Order: | Fabales |
| Family: | Fabaceae ⁄ Leguminosae |
| Genus: | Galactia |
| Species: | G. regularis |
| Binomial name | |
| Galactia regularis (L.) Britton, Sterns & Poggenb. | |
| |
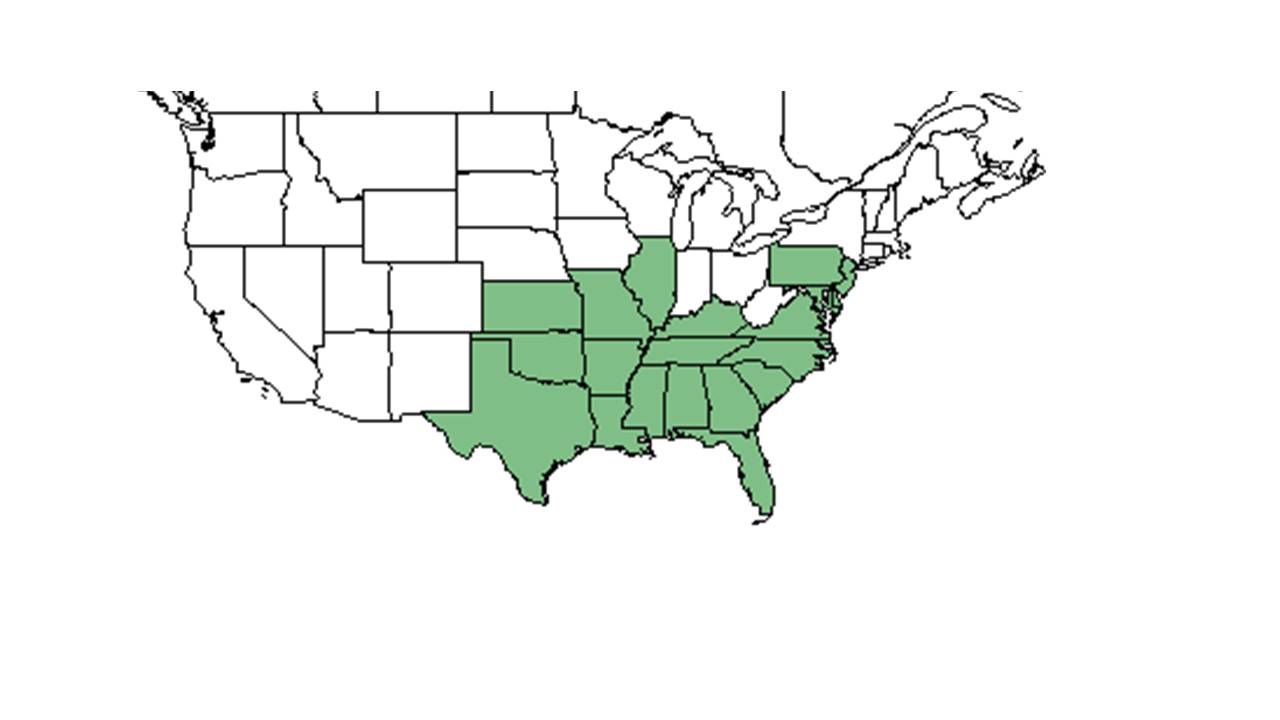
| Natural range of Galactia regularis from USDA NRCS Plants Database. | |
Common name: eastern milkpea
Contents
Taxonomic notes
Synonyms: Galactia volubilis (Linnaeus) Britton[1]
Varieties: G. macreei M.A. Curtis[1]
Description
G. regularis is a prostrate perennial with showy violet-purple flowers, frequently found climbing over bushes.[2] Stems have been observed to twine on low shrubs.[3] It has been documented as a prostrate structure. It is strongly paraheliotropic.[4]
Generally, the genus Galactia are "trailing or twining, climbing, perennial, herbaceous or woody vines or erect, perennial herbs or rarely shrubs. Leaves 1-pinnate, usually 3-foliolate (or rarely 1-,5-7-,9-folilolate); leaflets entire, petiolulate, stipellate. Racemes axillary, pedunculate with few to numerous, papilionaceous flowers borne solitary or 2-several at a node, each subtended by a bract and fusion of the 2 uppermost, with the laterals usually shorter than the uppermost and lowermost; petals usually red, purple, pink or white; stamens diadelphous or elsewhere occasionally monadelphous; ovary sessile or shortly stipitate. Legume oblong-linear to linear, few-many seeded, compressed, straight or slightly curbed, dehiscent with often laterally twisting valves."[5]
Specifically, for this species, G. regularis, they are "trailing, perennial herb with minutely appressed-pubescent to glabrate stems, 0.4-1.2 m long. Leaves 3-foliolate, rachis 3-18 mm long; leaflets oblong to elliptic or oblong-lanceolate, (1.2) 2-3.5 (5) cm long, glabrous, or nearly so, above and glabrous to appressed-pubescent beneath. Racemes with glabrous to appressed short-pubescent peduncles and rachises (1) 3-13 cm long; flowers few to many, each on a puberulent pedicel 1-5 mm long subtended by ovate to triangular-subulate bracts ca. 1 mm long; bractlets triangular to linear-subulate, 0.8-1.5 (3) mm long. Calyx glabrous or sparsely appressed-pubescent, tube 2-3.5 mm long, lobes 3-6 mm long; petals reddish purple, the standard 1.2-1.8 cm long. Legume densely appressed-pubescent, 2-5 cm long."[5]
Distribution
Galactia regularis is natively distributed from southeast Pennsylvania west to Missouri and Oklahoma, and south to southern Florida and southeastern Texas.[1] It occurs in pinelands and sandy woods from New York to Florida and Mississippi.[2] Occurs in a Pinus elliottii plantation in South Carolina.[6]
Ecology
Habitat
Generally, G. regularis can be found in woodlands and dry forests.[1] Galactia regularis has been documented in open sand ridges, open cedar glades, dry prairies, dry upland woods, along rocky banks, scrub oak-wiregrass ridges, shell ridge in a brackish marsh, dry grassy scrub border of a cypress swamp, open oak-hickory woods of a bog boarder, pine flatwoods at the edges of pond cypress wetland, edge of a floodplain woodland on a natural levee, and mature longleaf pine-wiregrass stand that is frequently burned.[3] It can be found in xeric areas with hot, wet summers and mild, dry winters.[7] G. regularis has been documented in pine sandhill communities.[8] It has also been observed in shrublands.[7] In disturbed habitats G. regularis has been found growing in areas of clay with sandstone that have been recently cleared and bulldozed along with developed locations. Soils range from sand to sandy loam.[3] At the Archbold Biological Station in central Florida, G. regularis was found to be associated with patches of rosemary scrub.[9] This species is also considered an indicator species of the Florida peninsula xeric sandhills habitat.[10] It preferentially grows in gaps that are created by fire disturbance.[11] G. regularis was found to increase its occurrence and abundance in response to soil disturbance by clearcutting and chopping in South Carolina. It has shown regrowth in reestablished native habitat that was disturbed by these practices.[12] However, it does not respond to soil disturbance by clearcutting and chopping in north Florida flatwoods forests.[13]
Species that have been associated with G. regularis are Elephantopus, Yucca, bahia grass, centipede grass, Galactia volubilis and Rhynchosia difformis.[3]
Galactia regularisis an indicator species for the Peninsula Xeric Sandhills community type as described in Carr et al. (2010).[14]
Phenology
This species generally flowers from July to September, and fruits from August to October.[1] G. regularis has been observed to flower from April through November.[15][3]
Seed dispersal
This species is thought to be dispersed by gravity. [16]
Seed bank and germination
Maximum germination was observed for G. regularis at the 80 degrees Celsius dry heat shock treatment. Wet heat (boiling water) treatments, however, resulted in 100% mortality of seeds.[17] Soil scarification seems to impede germination.[6] It readily resprouts and germinates from seed after fire.[18][19]
Fire ecology
In a field study of vegetation change in the Florida scrub, G. regularis increased in abundance post fire.[20] However, this increase may not be the direct result of fire. Heat shock germination may play a role in its post-fire recruitment.[17] The amount of G. regularis decreased after a spring burn; decreased slightly after a summer burn; and increased in the control plots.[21] A total of 24 plants in four new quadrants were recruited postburn study in the Florida scrub – Lake Wales Ridge area.[20] In previous studies conducted by Cushwa and his collegues determined that leguminous plants and their seeds respond best to hot, or high temperature, fires. Cuswha and his team also conducted laboratory tests indicating that the legume species will germinate the best when hit by moist heat, such as a prescribed fire being conducted on a day that is 80 degrees Fahrenheit.[21] As an herbaceous vine, the amount of groundcover of G. regularis increased slightly when the area has not been burned (controlled treatment). The amount of ground cover decreased from ~58 to 23% after a burn.[18] As well, a study by Dee and Menges found G. regularis to be positively associated with statistical significance growing in areas between 2 years and 8 years after a fire disturbance.[22]
Populations of Galactia regularis have been known to persist through repeated annual burning on the Pebble Hill plantation in north Florida.[23]
Pollination
Galactia regularis has been observed at the Archbold Biological Station to host thread-waisted wasps such as Trypargilum clavatum johannis (family Sphecidae), wasps such as Stenodynerus fundatiformis (family Vespidae), leafcutting bees from the family Megachilidae such as Anthidiellum notatum rufomaculatum, A. perplexum, Anthidium maculifrons, Coelioxys germana, C. sayi, Megachile albitarsis, M. brevis pseudobrevis, M. brimleyi, M. deflexa, M. exilis parexilis, M. georgica, M. integra, M. mendica, and M. petulans, bees from the family Apidae such as Apis mellifera, Bombus impatiens, and Svastra atripes, and sweat bees from the family Halictidae such as Augochlorella aurata, Augochloropsis metallica, A. sumptuosa, and Nomia maneei.[24] On occasion, this species occurred in White-tailed deer’s diet.[25]
Herbivory and toxicology
Seeds of G. regularis have been found in stomachs of the bobwhite and has been considered an important food.[2] At an Arkansas experiment station, this species is an important component of loblolly pine plantations for the diet of bobwhite quail in 0-year and 2-year stands.[26]
Diseases and parasites
G. regularis reposnded moderately resistant to a root-knotnematodes study. A nematode, M. incognita, showed an immune response to the root galls and egg masses in the 2004 study but only a highly resistant response in 2001.[27]
Conservation, cultivation, and restoration
This species is listed as extirpated by the Pennsylvania Department of Conservation and Natural Resources.[28]
Cultural use
Photo Gallery
References and notes
- ↑ 1.0 1.1 1.2 1.3 1.4 Weakley, A.S. 2020. Flora of the Southeastern United States. Edition of 20 October 2020. University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
- ↑ 2.0 2.1 2.2 Graham, E. H. (1941). Legumes for erosion control and wildlife. Washington, USDA
- ↑ 3.0 3.1 3.2 3.3 3.4 Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: July 2015. Collectors: Loran C. Anderson, H. E. Ahles, Tom Barnes, Michael B. Brooks, Robert W. Simons, Dianna Hall, R. Kral, R. K. Godfrey, Sidney McDaniel, R. A. Norris, H. R. Reed, Cecil R. Slaughter, Frankie Snow, A. E. Redford, C. Simon, A. A. Eaton, Robert L. Lazor, Bruce Hansen, JoAnn Hansen, W. A. Silveus, A. F. Clewell, Robert Blaisdell, O. Lakela, George R. Cooley, Richard J. Eaton, Daniel B. Ward, Paul O. Schallert, A. H. Curtiss. States and Counties: Florida: Bay, Brevard, Clay, Dixie, Duval, Escambia, Franklin, Flagler, Gadsden, Hillsborough, Jackson, Jefferson, Leon, Levy, Liberty, Madison, Nassau, Osceola, Putnam, Sarasota, St. Johns, Taylor, Wakulla, Washington. Georgia: Camden, Coffee, Grady. Mississippi: Pearl River, Oktibbeha. North Carolina: Alexander. South Carolina: Hampton. Virginia: Pulaski. Compiled by Tall Timbers Research Station and Land Conservancy.
- ↑ KMR observation at Pebble Hill Plantation, Georgia in July.
- ↑ 5.0 5.1 Radford, Albert E., Harry E. Ahles, and C. Ritchie Bell. Manual of the Vascular Flora of the Carolinas. 1964, 1968. The University of North Carolina Press. 643-4. Print.
- ↑ 6.0 6.1 Mou, P., R. H. Jones, et al. (2005). "Regeneration strategies, disturbance and plant interactions as organizers of vegetation spatial patterns in a pine forest." Landscape Ecology 20: 971-987.
- ↑ 7.0 7.1 Hawkes, C. V. and E. S. Menges (2003). "Effects of lichens on seedling emergence in a xeric Florida shrubland." Southeastern Naturalist 2: 223-234.
- ↑ Downer, M. R. (2012). Plant species richness and species area relationships in a Florida sandhill community. Integrative Biology. Ann Arbor, MI, University of South Florida. M.S.: 52.
- ↑ Quintana-Ascencio, P. F. and E. S. Menges (1996). "Inferring Metapopulation Dynamics from Patch-Level Incidence of Florida of Scrub Plants." Conservation Biology 10(4): 1210-1219.
- ↑ Carr, S. C., et al. (2010). "A Vegetation Classification of Fire-Dependent Pinelands of Florida." Castanea 75(2): 153-189.
- ↑ Young, C. C. and E. S. Menges. (1999). "Postfire gap-phase regeneration in scrubby flatwoods on the Lake Wales Ridge." Florida Scientist 62(1): 1-12.
- ↑ Cushwa, C.T. and M.B. Jones. (1969). Wildlife Food Plants on Chopped Areas in Piedmont South Carolina. Note SE-119. Asheville, NC: U.S. Department of Agriculture, Forest Service, Southeastern Forest Experiment Station. 4 pp.
- ↑ Moore, W.H., B.F. Swindel, and W.S. Terry. (1982). Vegetative Response to Clearcutting and Chopping in a North Florida Flatwoods Forest. Journal of Range Management 35(2):214-218.
- ↑ Carr, S.C., K.M. Robertson, and R.K. Peet. 2010. A vegetation classification of fire-dependent pinelands of Florida. Castanea 75:153-189.
- ↑ Nelson, G. PanFlora: Plant data for the eastern United States with emphasis on the Southeastern Coastal Plains, Florida, and the Florida Panhandle. www.gilnelson.com/PanFlora/ Accessed: 9 DEC 2016
- ↑ Kirkman, L. Katherine. Unpublished database of seed dispersal mode of plants found in Coastal Plain longleaf pine-grasslands of the Jones Ecological Research Center, Georgia.
- ↑ 17.0 17.1 Bolin, J. F. (2009). "Heat shock germination responses of three eastern North American temperate species." Castanea 74: 160-167. Cite error: Invalid
<ref>tag; name "bolin" defined multiple times with different content - ↑ 18.0 18.1 Reinhart, K. O. and E. S. Menges (2004). "Effects of re-introducing fire to a central Florida sandhill community." Applied Vegetation Science 7: 141-150.
- ↑ Freeman, J. E. and L. N. Kobziar (2011). "Tracking postfire successional trajectories in a plant community adapted to high-severity fire." Ecological Applications 21: 61-74.
- ↑ 20.0 20.1 Weekley, C.W. and E.S. Menges. 2003. Species and vegetation responses to prescribed fire in a long-unburned, endemic-rich Lake Wales ridge scrub. J. Torrey Bot. Soc. 130: 265-282. Bolin, J. F. (2009). "Heat shock germination responses of three eastern North American temperate species." Castanea 74: 160-167.
- ↑ 21.0 21.1 Cushwa, C. T., M. Hopkins, et al. (1970). Response of legumes to prescribed burns in loblolly pine stands of the South Carolina Piedmont. Asheville, NC, USDA Forest Service, Research Note SE-140: 6.
- ↑ Dee, J. R. and E. S. Menges (2014). "Gap ecology in the Florida scrubby flatwoods: effects of time-since-fire, gap area, gap aggregation and microhabitat on gap species diversity." Journal of Vegetation Science 25(5): 1235-1246.
- ↑ Robertson, K.M. Unpublished data collected from Pebble Hill Fire Plots, Pebble Hill Plantation, Thomasville, Georgia.
- ↑ Deyrup, M.A. and N.D. 2015. Database of observations of Hymenoptera visitations to flowers of plants on Archbold Biological Station, Florida, USA.
- ↑ Gee, K. L., M. D. Porter, et al. (1994). White-tailed deer : their foods and management in the cross timbers. Ardmore, OK, Samuel Roberts Noble Foundation.
- ↑ Sweeney, J. M., et al. (1981). Bobwhite quail food in young Arkansas loblolly pine plantations. Arkansas Experiment Station bulletin 852. Fayetteville, AR, University of Arkansas, Divisionn of Agriculture, Agricultural Experiment Station.
- ↑ Quesenberry, K. H., J. M. Dampier, et al. (2008). "Response of native southeastern U.S. legumes to root-knot nematodes." Crop Science 48: 2274-2278.
- ↑ USDA, NRCS. (2016). The PLANTS Database (http://plants.usda.gov, 13 May 2019). National Plant Data Team, Greensboro, NC 27401-4901 USA.
