Galactia regularis
| Galactia regularis | |
|---|---|

| |
| Photo was taken by Gil Nelson | |
| Scientific classification | |
| Kingdom: | Plantae |
| Division: | Magnoliophyta - Flowering plants |
| Class: | Magnoliopsida – Dicotyledons |
| Order: | Fabales |
| Family: | Fabaceae ⁄ Leguminosae |
| Genus: | Galactia |
| Species: | G. regularis |
| Binomial name | |
| Galactia regularis (L.) Britton, Sterns & Poggenb. | |
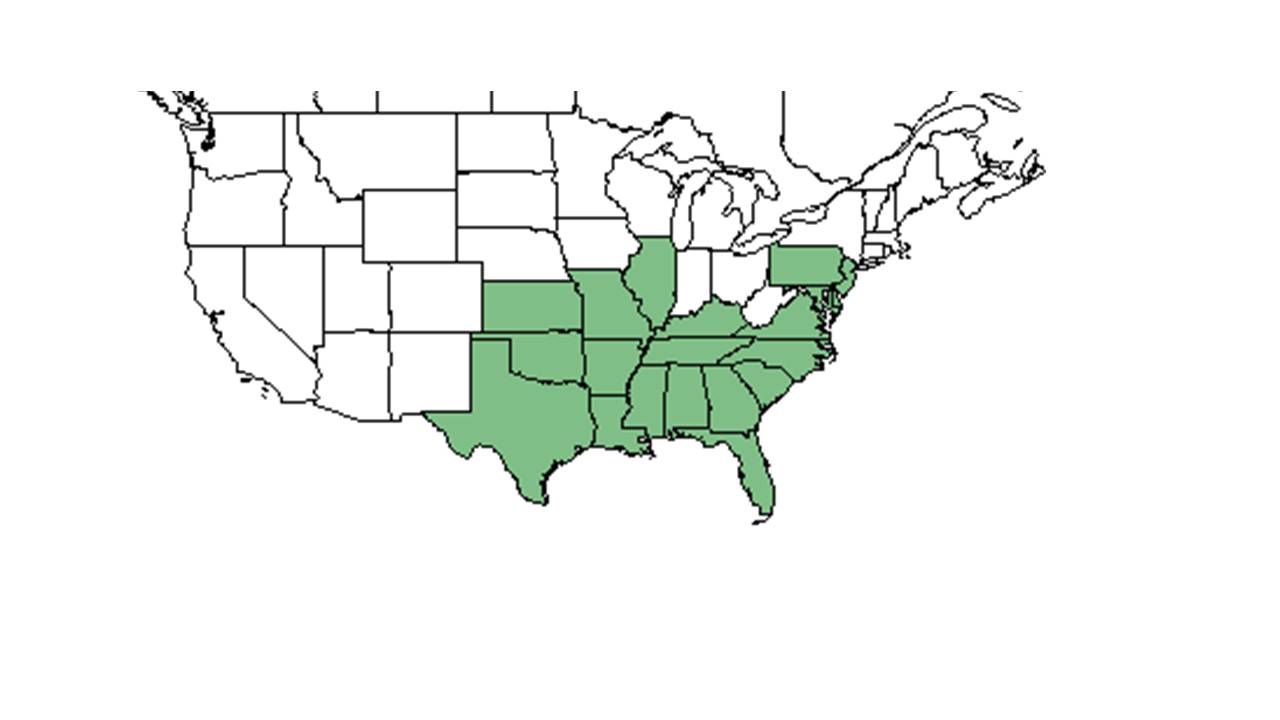
| |
| Natural range of Galactia regularis from USDA NRCS Plants Database. | |
Common name: eastern milkpea
Contents
Taxonomic notes
Synonyms: Galactia volubilis (Linnaeus) Britton; G. macreei M.A. Curtis; G. volubilis
Description
G. regularis is a prostrate perennial with showy violet-purple flowers, frequently found climbing over bushes (Graham 1941). Stems have been observed to twine on low shrubs (FSU Herbarium). It has been documented as a prostrate structure (FSU Herbarium). It is strongly paraheliotropic,[1]
Generally, the genus Galactia are "trailing or twining, climbing, perennial, herbaceous or woody vines or erect, perennial herbs or rarely shrubs. Leaves 1-pinnate, usually 3-foliolate (or rarely 1-,5-7-,9-folilolate); leaflets entire, petiolulate, stipellate. Racemes axillary, pedunculate with few to numerous, papilionaceous flowers borne solitary or 2-several at a node, ech subtended by a bract and fusion of the 2 uppermost, with the laterals usually shorter than the uppermost and lowermost; petals usually red, purple, pink or white; stamens diadelphous or elsewhere occasionally monadelphous; ovary sessile or shortly stipitate. Legume oblong-linear to linear, few-many seeded, compressed, straight or slightly curbed, dehiscent with often laterally twisting valves."- Radford et al 1964
Specifically, for this species, G. regularis, they are "trailing, perennial herb with minutely appressed-pubescent to glabrate stems, 0.4-1.2 m long. Leaves 3-foliolate, rachis 3-18 mm long; leaflets oblong to elliptic or oblong-lanceolate, (1.2) 2-3.5 (5) cm long, glabrous, or nearly so, above and glabrous to appressed-pubescent beneath. Racemes with glabrous to appressed short-pubescent peduncles and rachises (1) 3-13 cm long; flowers few to many, each on a puberulent pedicel 1-5 mm long subtended by ovate to triangular-subulate bracts ca. 1 mm long; bractlets triangular to linear-subulate, 0.8-1.5 (3) mm long. Calyx glabrous or sparsely appressed-pubescent, tube 2-3.5 mm long, lobes 3-6 mm long; petals reddish purple, the standard 1.2-1.8 cm long. Legume densely appressed-pubescent, 2-5 cm long, 4-5 cm broad." - Radford et al 1964
Distribution
It occurs in pinelands and sandy woods from New York to Florida and Mississippi (Graham 1941). Occurs in a Pinus elliottii plantation in South Carolina (Mou et. al 2005).
Ecology
Habitat
Galactia regularis has been documented in open sand ridges, open cedar glades, dry prairies, dry upland woods, along rocky banks, scrub oak-wiregrass ridges, shell ridge in a brackish marsh, dry grassy scrub border of a cypress swamp, open oak-hickory woods of a bog boarder, pine flatwoods at the edges of pond cypress wetland, edge of a floodplain woodland on a natural levee, and mature longleaf pine-wiregrass stand that is frequently burned (FSU Herbarium). It can be found in xeric areas with hot, wet summers and mild, dry winters (Hawkes and Menges 2003). G. regularis has been documented in pine sandhill communities (Downer 2012). It has also been observed in shrublands (Hawkes and Menges 2003).
In human impacted areas, G. regularis has been found growing in areas of clay with sandstone that have been recently cleared and bulldozed along with developed locations (FSU Herbarium).
Soils range from sand to sandy loam.
Species that have been associated with G. regularis are Elephantopus, Yucca, bahia grass, centipede grass, Galactia volubilis and Rhynchosia difformis (FSU Herbarium).
Phenology
Flowers June through November (FSU Herbarium).
Seed bank and germination
Maximum germination was observed for G. regularis at the 80 degrees Celsius dry heat shock treatment. Wet heat (boiling water) treatments, however, resulted in 100% mortality of seeds (Bolin 2009). Soil scarification seems to impede germination (Mou et. al 2005). It reproduces by resprouting after fire (Reinhart and Menges 2004).
Fire ecology
In a field study of vegetation change in the Florida scrub, G. regularis increased in abundance post fire (Weakley and Menges 2003). However, this increase may not be the direct result of fire. Heat shock germination may play a role in its post-fire recruitment (Bolin 2009). The amount of G. regularis decreased after a spring burn; decreased slightly after a summer burn; and increased in the control plots (Cushwa et al 1970). A total of 24 plants in four new quadrants were recruited postburn study in the Florida scrub – Lake Wales Ridge area (Weakley and Menges 2003).
In previous studies conducted by Cushwa and his collegues determined that leguminous plants and their seeds respond best to hot, or high temperature, fires. Cuswha and his team also conducted laboratory tests indicating that the legume species will germinate the best when hit by moist heat, such as a prescribed fire being conducted on a day that is 80 degrees Celsius (Cushwa et al 1970).
As an herbaceous vine, the amount of groundcover of G. regularis increased slightly when the area has not been burned (controlled treatment). The amount of ground cover decreased from ~58 to 23% after a burn (Reinhart and Menges 2004).
Pollination
The following Hymenoptera families and species were observed visiting flowers of Galactia regularis at Archbold Biological Station (Deyrup 2015):
Apidae: Apis mellifera, Bombus impatiens, Svastra atripes
Halictidae: Augochlorella aurata, Augochloropsis metallica, A. sumptuosa, Nomia maneei
Megachilidae: Anthidiellum notatum rufomaculatum, A. perplexum, Anthidium maculifrons, Coelioxys germana, C. sayi, Megachile albitarsis, M. brevis pseudobrevis, M. brimleyi, M. deflexa, M. exilis parexilis, M. georgica, M. integra, M. mendica, M. petulans
Sphecidae: Trypargilum clavatum johannis
Vespidae: Stenodynerus fundatiformis
Use by animals
On occasion, occurred in White-tailed deer’s diet (Gee et. al 1994). Seeds of G. regularis have been found in stomachs of the bobwhite and has been considered an important food (Graham 1941).
Diseases and parasites
G. regularis reposnded moderately resistant to a root-knotnematodes study. A nematode, M. incognita, showed an immune response to the root galls and egg masses in the 2004 study but only a highly resistant response in 2001 (Quesenberry et. al 2008).
Conservation and Management
Cultivation and restoration
Photo Gallery
References and notes
Bolin, J. F. (2009). "Heat shock germination responses of three eastern North American temperate species." Castanea 74: 160-167.
Cushwa, C. T., M. Hopkins, et al. (1970). Response of legumes to prescribed burns in loblolly pine stands of the South Carolina Piedmont. Asheville, NC, USDA Forest Service, Research Note SE-140: 6.
Deyrup, M.A. and N.D. 2015. Database of observations of Hymenoptera visitations to flowers of plants on Archbold Biological Station, Florida, USA.
Downer, M. R. (2012). Plant species richness and species area relationships in a Florida sandhill community. Integrative Biology. Ann Arbor, MI, University of South Florida. M.S.: 52.
Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: July 2015. Collectors: Loran C. Anderson, H. E. Ahles, Tom Barnes, Michael B. Brooks, Robert W. Simons, Dianna Hall, R. Kral, R. K. Godfrey, Sidney McDaniel, R. A. Norris, H. R. Reed, Cecil R. Slaughter, Frankie Snow, A. E. Redford, C. Simon, A. A. Eaton, Robert L. Lazor, Bruce Hansen, JoAnn Hansen, W. A. Silveus, A. F. Clewell, Robert Blaisdell, O. Lakela, George R. Cooley, Richard J. Eaton, Daniel B. Ward, Paul O. Schallert, A. H. Curtiss. States and Counties: Florida: Bay, Brevard, Clay, Dixie, Duval, Escambia, Franklin, Flagler, Gadsden, Hillsborough, Jackson, Jefferson, Leon, Levy, Liberty, Madison, Nassau, Osceola, Putnam, Sarasota, St. Johns, Taylor, Wakulla, Washington. Georgia: Camden, Coffee, Grady. Mississippi: Pearl River, Oktibbeha. North Carolina: Alexander. South Carolina: Hampton. Virginia: Pulaski. Compiled by Tall Timbers Research Station and Land Conservancy.
Gee, K. L., M. D. Porter, et al. (1994). White-tailed deer : their foods and management in the cross timbers. Ardmore, OK, Samuel Roberts Noble Foundation.
Graham, E. H. (1941). Legumes for erosion control and wildlife. Washington, USDA
Hawkes, C. V. and E. S. Menges (2003). "Effects of lichens on seedling emergence in a xeric Florida shrubland." Southeastern Naturalist 2: 223-234.
Mou, P., R. H. Jones, et al. (2005). "Regeneration strategies, disturbance and plant interactions as organizers of vegetation spatial patterns in a pine forest." Landscape Ecology 20: 971-987.
Quesenberry, K. H., J. M. Dampier, et al. (2008). "Response of native southeastern U.S. legumes to root-knot nematodes." Crop Science 48: 2274-2278.
Radford, Albert E., Harry E. Ahles, and C. Ritchie Bell. Manual of the Vascular Flora of the Carolinas. 1964, 1968. The University of North Carolina Press. 643-4. Print.
Reinhart, K. O. and E. S. Menges (2004). "Effects of re-introducing fire to a central Florida sandhill community." Applied Vegetation Science 7: 141-150.
Weakley, C.W. and E.S. Menges. 2003. Species and vegetation responses to prescribed fire in a long-unburned, endemic-rich Lake Wales ridge scrub. J. Torrey Bot. Soc. 130: 265-282. Bolin, J. F. (2009). "Heat shock germination responses of three eastern North American temperate species." Castanea 74: 160-167.
