Andropogon virginicus
| Andropogon virginicus | |
|---|---|

| |
| Photo by the Atlas of Florida Plants Database | |
| Scientific classification | |
| Kingdom: | Plantae |
| Division: | Magnoliophyta - Flowering plants |
| Class: | Liliopsida - Moncots |
| Order: | Cyperales |
| Family: | Poaceae |
| Genus: | Andropogon |
| Species: | A. virginicus |
| Binomial name | |
| Andropogon virginicus L | |
| |
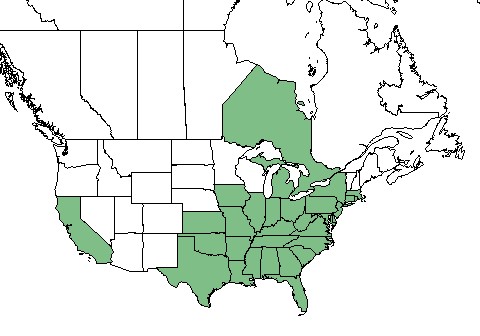
| Natural range of Andropogon virginicus from USDA NRCS Plants Database. | |
Common Name(s): Smooth Bluestem, Deceptive Bluestem, Old-field Broomstraw, Broomsedge, Sedge Grass, Sage Grass[1], Broomsedge Bluestem[2]
Contents
Taxonomic Notes
Synonyms: none.[3]
Varieties: A. virginicus var. virginicus; A. virginicus var. decipiens.[3].
Description
Andropogon virginicus is a monoecious warm season perennial graminoid.[2] It reaches 3-6 ft (0.91-1.83 m) in height and bunches together producing clumps (i.e. bunchgrass).[4] The leaves vary from partially folded to flat, reaching lengths of 10 to 15 inches and 1/8 inch in width. The plant itself is a pale greenish yellow color with basal leaf sheaths than can be colorless or yellow. It produces seeds on the upper half of the plant, and the inflorescence has a silvery color in the sunlight from a distance.[2] The fruits of A. virginicus are classified as an achene and caryopsis.[4] Peduncles and spikelets are short, and anthers are often marcescent within the spikelet.[5]
Distribution
Its primary distribution ranges from eastern Texas, Oklahoma, and Kansas eastward to the coast of the Atlantic Ocean and as far north as Illinois, Michigan, New York, Massachusetts.[2] It is also found in Puerto Rico, Ontario Canada, California, and was introduced in Hawaii.[2][6][7]
Ecology
Habitat
Andropogon virginicus is commonly found in longleaf pine savannas, savannas, flatwoods, maritime wet grasslands, disturbed pinelands, other wetlands, bogs, old fields, roadbanks, and disturbed sites[1][8] with sandy low fertility soils.[9] With such a wide range of habitats, A. virginicus has been shown to adapt to ecotones of varying water availability; in granite outcrops A. virginicus is more resistant to water loss than in old field habitat.[10] In old field succession, A. virginicus has been found to become dominant between the 4th and 5th year of succession and remains present in later stages.[11] Similar results in Mississippi show A. virginicus establishing in the 5th year of succession and remaining through the 15th year of the 16 year study.[12]
A. virginicus is also known for its allelopathic effects on surrounding organisms. In Oklahoma, studies have shown inhibition of seedling growth in species in the pioneering (e.g. Amaranthus palmeri, Bromus japonicus), primary (e.g. Amaranthus retroflexus), and secondary (e.g. Aristida oligantha, Andropogon scoparius) stages of old field succession. It also inhibits growth and nodulation legumes, including Lespedeza stipulacea and Trifolium repens. Inhibition has also been documented in free living and symbiotic nitrogen fixing bacteria (i.e. Azotobacter and Rhizobium). Such inhibitions may work to keep nitrogen availability low, thereby engineering its environment to keep its competative advantage over other species.[9]
A. virginicus responds both negatively, positively, and not at all to soil disturbance by roller chopping in Northwest Florida sandhills.[13] However, it responds negatively to soil disturbance by roller chopping in South Florida.[14] However, A. virginicus responds positively to soil disturbance by clearcutting and roller chopping in North Florida.[15] It also responds positively to soil disturbance by clearcutting and chopping in North Florida flatwoods forests.[16] A. virginicus responds positively and negatively to soil disturbance by roller chopping and disturbance by a KG blade in East Texas Loblolly Pine-Hardwood Forests.[17]
Associated species: Andropogon sp., Bidens sp., Chamaecrista fasciculata, Cyerus sp., Erianthus brevibarbus, Euphorbia sp., Ilex coriacea, Ilex glabra, Lycopodium carolinianum, L. alopecuroides, Nyssa biflora, Pteridium aquilinum, Scizachyrium sp., Sorghastrum sp., Spartina patens, Sporobolous vaginiflorus, Tridens strictus, Vaccinium myrsinites, and Woodwardia sp.[8]
Andropogon virginicus is frequent and abundant in the Panhandle Xeric Sandhills, North Florida Longleaf Woodlands, Clayhill Longleaf Woodlands, Panhandle Silty Longleaf Woodlands, Xeric Flatwoods, North Florida Mesic Flatwoods, Central Florida Flatwoods/Prairies, and Wet Depression Prairies community types as described in Carr et al. (2010).[18]
Phenology
A. virginicus has been observed flowering and fruiting between the months of September and December, producing a yellow flower.[8][4][19] Flowering usually occurs at, or shortly after, dawn as it is triggered by light.[19] Seeds develop fine hairs along the racemes giving raceme clusters a broom-like appearance.[4]
Seed dispersal
This species is thought to be dispersed by wind. [20] These seeds being wind dispersed likely contributes towards this plant's domination of colonized sites and its spread to new sites.[21] As well, their long spreading hairs on the fruit provide an increased aerodynamic drag, and it has a greater dispersal range due to the overall plant height.[5]
Seed bank and germination
In Hawaii where A. virginicus is exotic, the abundance of seeds in the seed rain and the seed bank vary between seasons. Persistent seeds were found ranging from around 90-130 seeds m-2 which allows for this species to wait for disturbances and then quickly colonize the area.[22] Seed banks in a New Jersey old field succession site showed seed to primarily occurr in the top 3 cm of soil and to reach densities around 350 seeds m-2. It is also worth noting seedlings from A. virginicus seeds did germinate from the seed bank until the 5th year of succession.[23] This appearance in the seed bank coincides with the establishment of A. virginicus on the 4th and 5th years of other successional studies in Illinois and Mississippi.[11][12] Although seeds enter the seed bank during October and November, germination does not begin until mid January and may require a cold period prior to germination.[24]
Fire ecology
A review of early studies suggests A. virginicus can be nearly eliminated by fires.[25] However, more recent studies[26] suggest A. virginicus is a pyrophyte that helps create a fire driven system by adding fuel to the fire and thereby promoting increases in fire frequency and size.[7] A possible fire adaptation is the hygroscopic awn and callus hairs that together work the spikelet fruit into the soil so that it is potentially more protected from fire.[5] It maintains extremely high dead:live biomass ratios during most of the year at 80-90%. Additionally, it can burn at high relative humidity (85-90%) and fuel moisture (20-25%). When burned, it recovers quickly and with increased vigor.[27] Following a November 28, 2015 burn, resprouts were noted 38 days after the fire.[28] Densities peak in the second or third growing season following burns and then decline, being replaced by pines.[24][29] One study noted that A. virginicus may become abundant after fire in pocosins through rapid colonization.[30]
Use by animals
A. virginicus is not an important cattle forage, but still provides food for grazing between the spring and early summer, and nutritional quality quickly decreases as summer continues.[2] It is also a source of food for other large mammals, like white-tailed deer.[31] The seeds are utilized by small birds only when other sources of food are limited, and broomsedge bluestem provides cover for ground nesting birds like turkey and quail. A. virginicus is a larval host for the Zabulon skipper butterfly (Poanes zabulon).[2]
Conservation, cultivation, and restoration
A. virginicus is considered a noxious weed in Hawaii submontane zones[27] where it was introduced[2] and spread in the 1960's and 1970's.[7] Part of its successful invasion could be due to its ability to quickly invade disturbed lands, including those recently burned.[4] Management suggestions include mowing as an effective method, where this reduces weed competition and keeps from covering small grass seedlings. Another option is by selective herbicides, such as imazapic herbicides, that control broadleaf grasses and are recommended for use on warm season grasses; best time of application for the most success is in the spring. Where A. virginicus is considered a pasture weed, avoid overgrazing other desirable grasses since it increases in numbers when other desirable grasses are selectively grazed.[2] The only commercially available cultivar of A. virginicus is 'Silver Beauty'. Overall, this bluestem is used as an ornamental for golf courses and residential landscaping.[2]
Cultural use
Photo Gallery
References and notes
- ↑ 1.0 1.1 Weakley A. S.(2015). Flora of the Southern and Mid-Atlantic States. Chapel Hill, NC: University of North Carolina Herbarium.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 2.9 USDA, NRCS. (2016). The PLANTS Database (http://plants.usda.gov, 30 November 2017). National Plant Data Team, Greensboro, NC 27401-4901 USA.
- ↑ 3.0 3.1 Weakley, A.S. 2015. Flora of the southern and mid-atlantic states. Working Draft of 21 May 2015. University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
- ↑ 4.0 4.1 4.2 4.3 4.4 Plant database: Andropogon virginicus. (13 December 2017).Lady Bird Johnson Wildflower Center. URL: https://www.wildflower.org/plants/search.php?search_field=&newsearch=true
- ↑ 5.0 5.1 5.2 Campbell, C. S. (1983). "Systematics of the Andropogon virginicus complex (Gramineae)." Journal of the Arnold Arboretum 64(2): 171-254.
- ↑ Catling P. M., Reznicek A. A., Riley J. L. (1977). Some new and interesting grass records from southern Ontario. Canadian Field-Naturalist 91(4):350-359.
- ↑ 7.0 7.1 7.2 "Smith C. W. and Tunison J. T. (1992). Fire and alien plants in Hawai'i: Research and management implications for native ecosystems. Alien plant invasions in native ecosystems of Hawaii: management and research. Cooperative National Park Resources Studies Unit, Honolulu 394-408.
- ↑ 8.0 8.1 8.2 Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: March 2019. Collectors: Loran C. Anderson, Wilson Baker, Joe Beck, K. Blum, Glen E. Breland, C. S. Campbell, A. F. Clewell, George R. Cooley, A. H. Curtiss, J. Dwyer, R. J. Eaton, Angus Gholson, R. K. Godfrey, David Hall, Brenda Herring, Ann F. Johnson, Walter Judd, Palmer Kinser, R. Komarek, R. Kral, Kurz, Olga Lakela, Robert L. Lazor, S. W. Leonard, Travis MacClendon, J. S. McCorkle, J. B. McFarlin, Richard S. Mitchell, Herbert Monoson, Neal Morar, John Morrill, Annie Schmidt, Gary Schultz, Silvus, Cecil R. Slaughter, Jason R. Swallen, Thompson, Amanda R. Travis, and Edwin L. Tyson. States and Counties: Florida: Baker, Bay, Calhoun, Clay, Collier, Columbia, Dixie, Duval, Franklin, Gadsden, Hamilton, Hernando, Highlands, Holmes, Indian River, Jackson, Jefferson, Lafayette, Lee, Leon, Liberty, Madison, Martin, Nassau, Okaloosa, Orange, Pasco, Putnam, Santa Rosa, Sarasota, St Johns, Taylor, Volusia, Wakulla, Walton, and Washington. Georgia: Baker and Thomas.
- ↑ 9.0 9.1 Rice E. L. (1972). Allelopathic effects of Andropogon virginicus and its persistence in old fields. American Journal of Botany 59(7):752-755.
- ↑ Chapman R. H. and Jones, Jr. S. B. (1975). Ecotypic differentiation in Andropogon virginicus (Gramineae). Bulletin of the Torrey Botanical Club 102(4):166-171.
- ↑ 11.0 11.1 Bazzaz F. A. (1975). Plant species diversity in old-field successional ecosystems in southern Illinois. Ecology 56:485-488.
- ↑ 12.0 12.1 Battaglia L. L., Minchin P. R., and Pritchett D. W. (2002). Sixteen years of old-field succession and reestablishment of a bottomland hardwood forest in the lower Mississippi alluvial valley. Wetlands 22(1):1-17.
- ↑ Hebb, E.A. (1971). Site Preparation Decreases Game Food Plants in Florida Sandhills. The Journal of Wildlife Management 35(1):155-162.
- ↑ Lewis, C.E. (1970). Responses to Chopping and Rock Phosphate on South Florida Ranges. Journal of Range Management 23(4):276-282.
- ↑ Lewis, C.E., G.W. Tanner, and W.S. Terry. (1988). Plant responses to pine management and deferred-rotation grazing in north Florida. Journal of Range Management 41(6):460-465.
- ↑ Moore, W.H., B.F. Swindel, and W.S. Terry. (1982). Vegetative Response to Clearcutting and Chopping in a North Florida Flatwoods Forest. Journal of Range Management 35(2):214-218.
- ↑ Stransky, J.J., J.C. Huntley, and Wanda J. Risner. (1986). Net Community Production Dynamics in the Herb-Shrub Stratum of a Loblolly Pine-Hardwood Forest: Effects of CLearcutting and Site Preparation. Gen. Tech. Rep. SO-61. New Orleans, LA: U.S. Dept of Agriculture, Forest Service, Southern Forest Experiment Station. 11 p.
- ↑ Carr, S.C., K.M. Robertson, and R.K. Peet. 2010. A vegetation classification of fire-dependent pinelands of Florida. Castanea 75:153-189.
- ↑ 19.0 19.1 Campbell C. S. (1982). Cleistogamy in Andropogon L. (Gramineae). American Journal of Botany 69(10):1625-1635.
- ↑ Kirkman, L. Katherine. Unpublished database of seed dispersal mode of plants found in Coastal Plain longleaf pine-grasslands of the Jones Ecological Research Center, Georgia.
- ↑ Campbell C. S. (1983). Wind dispersal of some North American species of Andropogon (Gramineae). Rhodora 85(841):65-72.
- ↑ Drake D. R. (1998). Relationships among the seed rain, seed bank, and vegetation of a Hawaiian forest. Journal of Vegetation Science. 9(1):103-112.
- ↑ Leck M. A. and Leck C. F. (1998). A ten-year seed bank study of old field succession in central New Jersey. The Journal of the Torrey Botanical Society 125(1):11-32.
- ↑ 24.0 24.1 Keever C. (1950). Causes of succession on old fields of the Piedmont, North Carolina. Ecological Monographs 20(3):229-250.
- ↑ Garren K. H. (1943). Effects of fire on vegetation of the southeastern United States. Botanical Review 9(9):617-654.
- ↑ Hodgkins E. J. (1958). Effects of fire on undergrowth vegetation in upland southern pine forests. Ecology 39(1):36-46.
- ↑ 27.0 27.1 Hughes F., Vitousek P. M., and Tunison T. (1991). Alien grass invasion and fire in the seasonal submontane zone of Hawai'i. Ecology 72(2):743-747.
- ↑ Observation by Edwin Bridges in northern Highlands County, FL, January 5, 2016, posted to Florida Flora and Ecosystematics Facebook Group January 6, 2016.
- ↑ Lemon P. C. (1949). Successional responses of herbs in the longleaf-slash pine forest after fire. Ecology 30(2):135-145.
- ↑ Bolin, J. F. (2007). "Seed bank response to wet heat and the vegetation structure of a Virginia pocosin." Journal of the Torrey Botanical Society 134: 80-88.
- ↑ Atwood, E. L. (1941). "White-tailed deer foods of the United States." The Journal of Wildlife Management 5(3): 314-332.