Difference between revisions of "Seymeria pectinata"
| Line 36: | Line 36: | ||
===Habitat=== <!--Natural communities, human disturbed habitats, topography, hydrology, soils, light, fire regime requirements for removal of competition, etc.--> | ===Habitat=== <!--Natural communities, human disturbed habitats, topography, hydrology, soils, light, fire regime requirements for removal of competition, etc.--> | ||
In the Coastal Plain in Florida and Georgia, ''S. pectinata'' can occur in open woodlands, flat longleaf pine-wiregrass communities, sand live oak groves, and sandridges.<ref name="FSU Herbarium">Florida State University Robert K. Godfrey Herbarium database. URL: [http://herbarium.bio.fsu.edu http://herbarium.bio.fsu.edu]. Last accessed: July 2015. Collectors: Loran C. Anderson, R. A. Norris, Robert K. Godfrey, Cecil R Slaughter. States and Counties: Florida: Duval, Franklin, Gadsden, Leon, Liberty, Madison. Georgia: Grady, Thomas. Compiled by Tall Timbers Research Station and Land Conservancy.</ref> It also occurs along roads. Soils include sandy soil and loamy sand.<ref name="FSU Herbarium"/> | In the Coastal Plain in Florida and Georgia, ''S. pectinata'' can occur in open woodlands, flat longleaf pine-wiregrass communities, sand live oak groves, and sandridges.<ref name="FSU Herbarium">Florida State University Robert K. Godfrey Herbarium database. URL: [http://herbarium.bio.fsu.edu http://herbarium.bio.fsu.edu]. Last accessed: July 2015. Collectors: Loran C. Anderson, R. A. Norris, Robert K. Godfrey, Cecil R Slaughter. States and Counties: Florida: Duval, Franklin, Gadsden, Leon, Liberty, Madison. Georgia: Grady, Thomas. Compiled by Tall Timbers Research Station and Land Conservancy.</ref> It also occurs along roads. Soils include sandy soil and loamy sand.<ref name="FSU Herbarium"/> | ||
| + | |||
| + | ''S. pectinata'' became absent or decreased in occurrence in response to soil disturbance by agriculture in some parts of southwest Georgia pinelands. However, in other areas of southwest Georgia this species increased its occurrence in response to agriculture. It has shown both regrowth and resistance to regrowth in reestablished pine habitat that was disturbed by agriculture.<ref> Ostertag, T. E. and K. M. Robertson. 2007. A comparison of native versus old-field vegetation in upland pinelands managed with frequent fire, South Georgia, USA. Tall Timbers Fire Ecology Conference Proceedings 23: 109-120.</ref> | ||
Associated species include longleaf pine, wiregrass, sand live oak, and sand pine.<ref name="FSU Herbarium"/> | Associated species include longleaf pine, wiregrass, sand live oak, and sand pine.<ref name="FSU Herbarium"/> | ||
Revision as of 12:40, 24 June 2021
| Seymeria pectinata | |
|---|---|

| |
| Photo taken by Kevin Robertson | |
| Scientific classification | |
| Kingdom: | Plantae |
| Division: | Magnoliophyta – Flowering plants |
| Class: | Magnoliopsida – Dicotyledons |
| Order: | Scrophulariales |
| Family: | Scrophulariaceae |
| Genus: | Seymeria |
| Species: | S. pectinata |
| Binomial name | |
| Seymeria pectinata Pursh | |
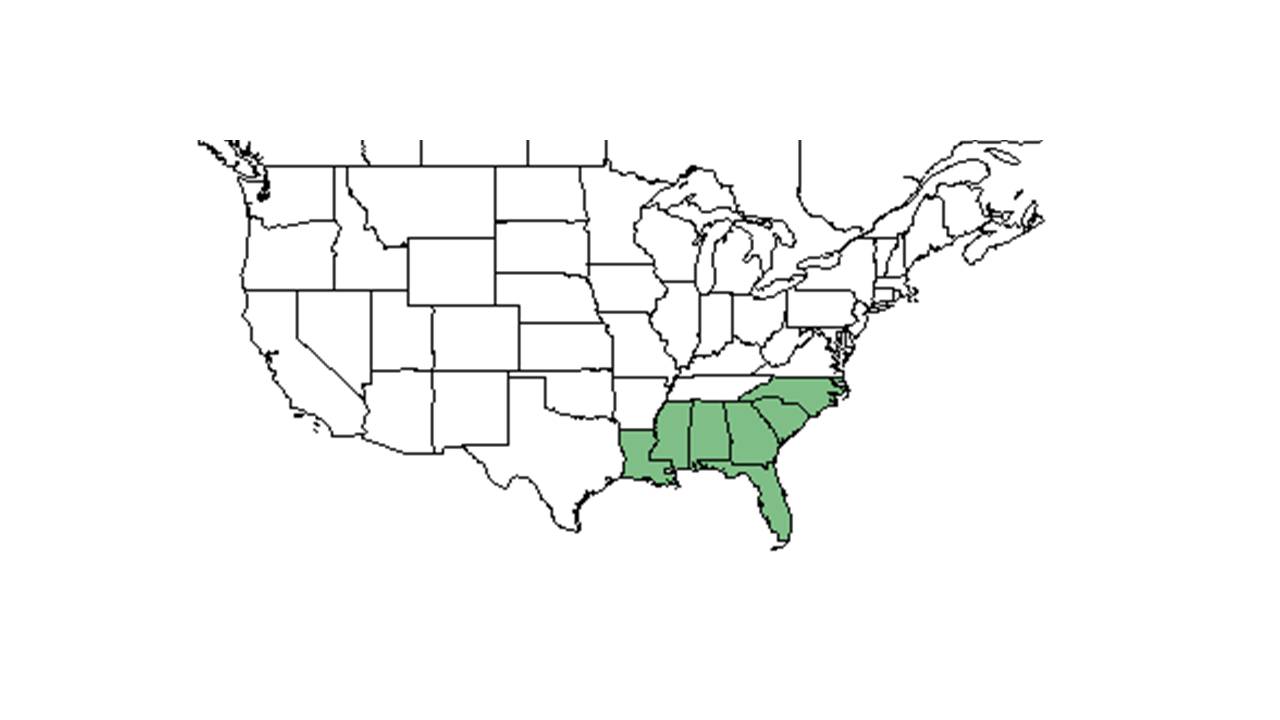
| |
| Natural range of Seymeria pectinata from USDA NRCS Plants Database. | |
Common names: Piedmont blacksenna, Combleaf seymeria
Contents
Taxonomic notes
Synonyms: Seymeria pectinata Pursh ssp. pectinata; Afzelia pectinata (Pursh) Kuntze ssp. pectinata
Description
It can grow up to 75 centimeters tall and is covered with stiff nonglandular hairs. It is stiffly branched with the longest branches occurring at the base. Seed capsules are ovoid covered with glandular hairs; flowers are around 1 centimeter long and yellow with hairs.[1]
"Erect, often profusely branched, presumably parasitic, glandular-pubescent annuals. Leaves opposite, pinnately or bipinnately parted or divided into linear to filiform segments. Flowers axillary, solitary, the terminal racemes weakly differentiated. Calyx lobes 5, longer than the tube; corolla yellow, rotate, 9-10 mm long, nearly regular, the lobes 5, longer than the tube; stamens 4, exserted, filaments pubescent. Capsule ovoid, 4-6 mm long; seeds numerous, winged."[2]
"Plant moderately branched, the branches spreading, stem pubescence of mixed long and short spreading trichomes. Leaves mostly more than 1 cm long, segments linear, 1-2 mm wide. Calyx lobes 4-5 mm long. Capsule glandular-pubescent."[2]
Distribution
S. pectinata is much more restricted in its distribution than S. cassioides however, it has a broader host range. It often prefers drier sites than S. cassioides and on occasion they can be found growing together. There is no evidence of hybridization between the two species.[1]
Ecology
Habitat
In the Coastal Plain in Florida and Georgia, S. pectinata can occur in open woodlands, flat longleaf pine-wiregrass communities, sand live oak groves, and sandridges.[3] It also occurs along roads. Soils include sandy soil and loamy sand.[3]
S. pectinata became absent or decreased in occurrence in response to soil disturbance by agriculture in some parts of southwest Georgia pinelands. However, in other areas of southwest Georgia this species increased its occurrence in response to agriculture. It has shown both regrowth and resistance to regrowth in reestablished pine habitat that was disturbed by agriculture.[4]
Associated species include longleaf pine, wiregrass, sand live oak, and sand pine.[3]
Phenology
Seymeria pectinata flowers and fruits in September.[3]
Pollination and use by animals
Deyrup conducted a study and observed pollinators such as Augochlorella aurata, Augochloropsis sumptuosa, Dialictus placidensis, Anthidiellum notatum rufimaculatum, A. perplexum, Coelioxys sayi, Megachile brevis pseudobrevis, M. mendica, M. petulans, and Bombus impatiens, on S. pectinata (2002). Additionally, Seymeria pectinata has been observed at the Archbold Biological Station to host bees from the Halictidae family such as Augochlorella aurata, Augochloropsis sumptuosa, and Lasioglossum placidensis, as well as bees from the Megachilidae family such as Anthidiellum notatum rufomaculatum, Anthidiellum perplexum, Coelioxys sayi, Megachile brevis pseudobrevis, M. mendica, and M. petulans.[5]
Conservation, cultivation, and restoration
Cultural use
Photo Gallery
References and notes
Deyrup, Mark, Jayanthi Edirisinghe, and Beth Norden. 2002. The Diversity and Floral Hosts of Bees at the Archbold Biological Station, Florida (Hymenoptera: Apoidea). Insect Mundi 16.1-3: 87-120.
- ↑ 1.0 1.1 Musselman, Lytton J., and William F. Mann, Jr. "Root Parasites of Southern Forests." Southern Forest Experiment Station (1978.
- ↑ 2.0 2.1 Radford, Albert E., Harry E. Ahles, and C. Ritchie Bell. Manual of the Vascular Flora of the Carolinas. 1964, 1968. The University of North Carolina Press. 956. Print.
- ↑ 3.0 3.1 3.2 3.3 Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: July 2015. Collectors: Loran C. Anderson, R. A. Norris, Robert K. Godfrey, Cecil R Slaughter. States and Counties: Florida: Duval, Franklin, Gadsden, Leon, Liberty, Madison. Georgia: Grady, Thomas. Compiled by Tall Timbers Research Station and Land Conservancy.
- ↑ Ostertag, T. E. and K. M. Robertson. 2007. A comparison of native versus old-field vegetation in upland pinelands managed with frequent fire, South Georgia, USA. Tall Timbers Fire Ecology Conference Proceedings 23: 109-120.
- ↑ Deyrup, M.A. and N.D. 2015. Database of observations of Hymenoptera visitations to flowers of plants on Archbold Biological Station, Florida, USA.