Difference between revisions of "Lycopodiella alopecuroides"
(→Pollination and use by animals) |
HaleighJoM (talk | contribs) (→Ecology) |
||
| Line 36: | Line 36: | ||
===Habitat=== <!--Natural communities, human disturbed habitats, topography, hydrology, soils, light, fire regime requirements for removal of competition, etc.--> | ===Habitat=== <!--Natural communities, human disturbed habitats, topography, hydrology, soils, light, fire regime requirements for removal of competition, etc.--> | ||
''L. alopecuroides'' occurs in moist to wet semi-shaded soils.<ref name="FSU Herbarium">Florida State University Robert K. Godfrey Herbarium database. URL: [http://herbarium.bio.fsu.edu http://herbarium.bio.fsu.edu]. Last accessed: June 2014. Collectors: F. Almeda, Loran C. Anderson, K. Craddock Burks, A. H. Curtiss, Mary Davis, J. P. Gillespie Robert K. Godfrey, Richard D. Houk, C. Jackson, Michael R. Jenkins, Roy Komarek, R. Kral, O. Lakela, R. Lazor, S. W. Leonard, Sidney McDaniel, John T. Mickel, Leon Neel, James H. Peck, Alan R. Smith, E. Laurence Thurston, L. B. Trott, E.S. Ford, P.L. Redfearn, Jr., Allen G. Shuey, Travis MacClendon, Karen MacClendon, and Floyd Griffith. States and Counties: Florida: Alachua, Baker, Bay, Calhoun, Clay, Duval, Franklin, Gulf, Hillsborough, Jackson, Jefferson, Leon, Liberty, Manatee, Okaloosa, Santa Rosa, Wakulla, and Washington. Georgia: Grady and Thomas.</ref> Typical soil types range from sandy-peaty soils to loamy sand.<ref name="FSU Herbarium"/> This species can be found in several native habitats, but also in disturbed areas. Native habitat types include wet pine savanna,<ref name="Brewer et al 2011">Brewer, J. S., D. J. Baker, et al. (2011). "Carnivory in plants as a beneficial trait in wetlands." Aquatic Botany 94: 62-70.</ref> hillside bogs, seasonally inundated meadows, damp grassy palmetto woods, and on the edges of ponds, creeks, and titi thickets.<ref name="FSU Herbarium"/> It also occurs in disturbed habitat like powerline corridors, roadsides, railways, pine plantations, and borrow pits.<ref name="FSU Herbarium"/> Associated species include ''Ilex myrtifolia, Nyssa biflora, Pinus palutris, Aristida stricta, Cyrilla racemiflora, Juncus, Sarracenia, Rhynchospora, Xyris, Eriocaulon, Liquidambar styraciflua, Quercus laevis, Pinguicula, Kalmia hirsuta, Serenoa repens, Hypericum, Cliftonia,'' and ''Magnolia virginiana''.<ref name="FSU Herbarium"/> | ''L. alopecuroides'' occurs in moist to wet semi-shaded soils.<ref name="FSU Herbarium">Florida State University Robert K. Godfrey Herbarium database. URL: [http://herbarium.bio.fsu.edu http://herbarium.bio.fsu.edu]. Last accessed: June 2014. Collectors: F. Almeda, Loran C. Anderson, K. Craddock Burks, A. H. Curtiss, Mary Davis, J. P. Gillespie Robert K. Godfrey, Richard D. Houk, C. Jackson, Michael R. Jenkins, Roy Komarek, R. Kral, O. Lakela, R. Lazor, S. W. Leonard, Sidney McDaniel, John T. Mickel, Leon Neel, James H. Peck, Alan R. Smith, E. Laurence Thurston, L. B. Trott, E.S. Ford, P.L. Redfearn, Jr., Allen G. Shuey, Travis MacClendon, Karen MacClendon, and Floyd Griffith. States and Counties: Florida: Alachua, Baker, Bay, Calhoun, Clay, Duval, Franklin, Gulf, Hillsborough, Jackson, Jefferson, Leon, Liberty, Manatee, Okaloosa, Santa Rosa, Wakulla, and Washington. Georgia: Grady and Thomas.</ref> Typical soil types range from sandy-peaty soils to loamy sand.<ref name="FSU Herbarium"/> This species can be found in several native habitats, but also in disturbed areas. Native habitat types include wet pine savanna,<ref name="Brewer et al 2011">Brewer, J. S., D. J. Baker, et al. (2011). "Carnivory in plants as a beneficial trait in wetlands." Aquatic Botany 94: 62-70.</ref> hillside bogs, seasonally inundated meadows, damp grassy palmetto woods, and on the edges of ponds, creeks, and titi thickets.<ref name="FSU Herbarium"/> It also occurs in disturbed habitat like powerline corridors, roadsides, railways, pine plantations, and borrow pits.<ref name="FSU Herbarium"/> Associated species include ''Ilex myrtifolia, Nyssa biflora, Pinus palutris, Aristida stricta, Cyrilla racemiflora, Juncus, Sarracenia, Rhynchospora, Xyris, Eriocaulon, Liquidambar styraciflua, Quercus laevis, Pinguicula, Kalmia hirsuta, Serenoa repens, Hypericum, Cliftonia,'' and ''Magnolia virginiana''.<ref name="FSU Herbarium"/> | ||
| − | |||
<!--===Phenology===--> <!--Timing off flowering, fruiting, seed dispersal, and environmental triggers. Cite PanFlora website if appropriate: http://www.gilnelson.com/PanFlora/ --> | <!--===Phenology===--> <!--Timing off flowering, fruiting, seed dispersal, and environmental triggers. Cite PanFlora website if appropriate: http://www.gilnelson.com/PanFlora/ --> | ||
<!--===Seed dispersal===--> | <!--===Seed dispersal===--> | ||
| Line 42: | Line 41: | ||
===Fire ecology=== <!--Fire tolerance, fire dependence, adaptive fire responses--> | ===Fire ecology=== <!--Fire tolerance, fire dependence, adaptive fire responses--> | ||
This species occurs in habitat that is maintained by frequent or annual fire.<ref name="FSU Herbarium"/> | This species occurs in habitat that is maintained by frequent or annual fire.<ref name="FSU Herbarium"/> | ||
| − | + | <!--===Pollination===--> | |
| − | === | + | ===Herbivory and toxicology=== |
''Lycopodiella alopecuroides'' has been observed at the Archbold Biological Station to host sweat bees such as ''Augochlorella aurata'' (family Halictidae).<ref name="Deyrup 2015">Deyrup, M.A. and N.D. 2015. Database of observations of Hymenoptera visitations to flowers of plants on Archbold Biological Station, Florida, USA.</ref> | ''Lycopodiella alopecuroides'' has been observed at the Archbold Biological Station to host sweat bees such as ''Augochlorella aurata'' (family Halictidae).<ref name="Deyrup 2015">Deyrup, M.A. and N.D. 2015. Database of observations of Hymenoptera visitations to flowers of plants on Archbold Biological Station, Florida, USA.</ref> | ||
<!--===Diseases and parasites===--> | <!--===Diseases and parasites===--> | ||
Revision as of 18:52, 14 July 2022
| Lycopodiella alopecuroides | |
|---|---|

| |
| Photo taken by Gil Nelson | |
| Scientific classification | |
| Kingdom: | Plantae |
| Division: | Lycopodiophyta – Lycopods |
| Class: | Lycopodiopsida |
| Order: | Lycopodiales |
| Family: | Lycopodiaceae |
| Genus: | Lycopodiella |
| Species: | L. alopecuroides |
| Binomial name | |
| Lycopodiella alopecuroides (L.) Cranfill | |
| |
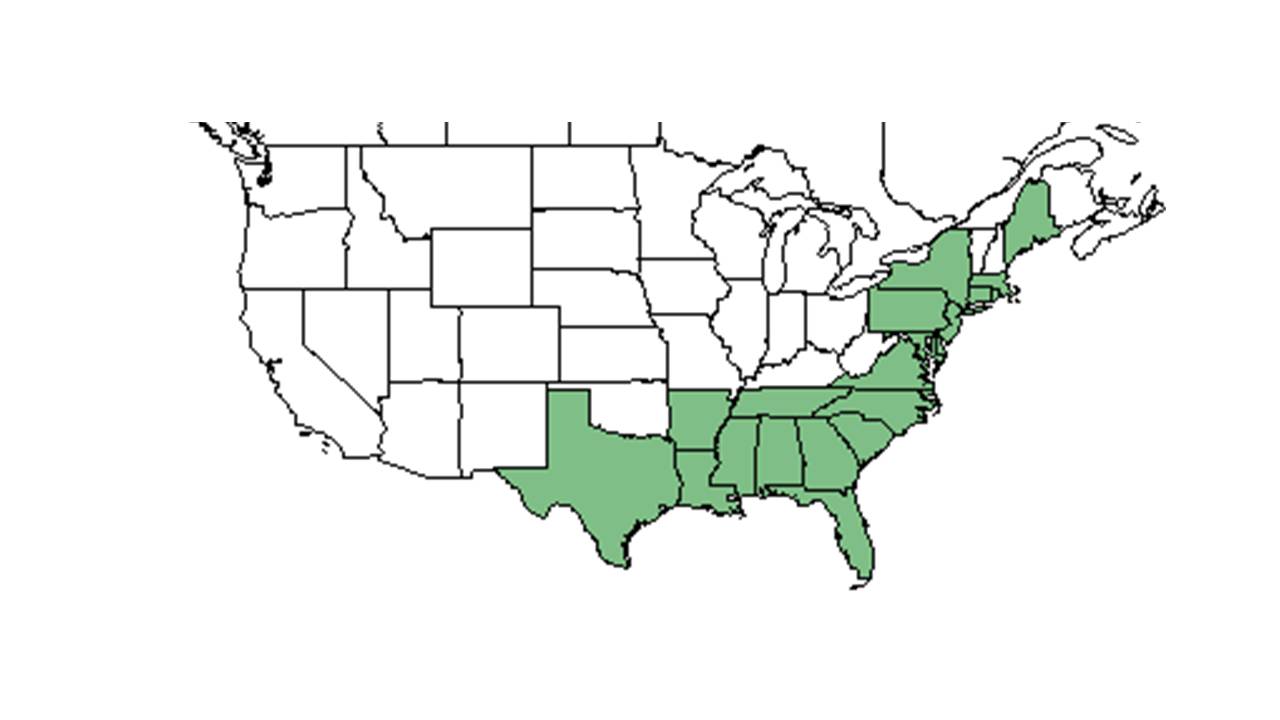
| Natural range of Lycopodiella alopecuroides from USDA NRCS Plants Database. | |
Common name: Foxtail clubmoss[1]
Contents
Taxonomic notes
Synonyms: Lycopodium alopecuroides Linnaeus.[1]
Varieties: none.[1]
Description
A description of Lycopodiella alopecuroides is provided in The Flora of North America.
"Stems subterranean or on the surface, branching dichotomously and ascending or dividing into horizontal and erect branches. Roots adventitious along the creeping stem. Leaves numerous, small, scale-or awn-like, to 13 mm long, appressed or spreading, sometimes partly fused with the branch, simple, entire or toothed, with a midrib only. Sporophylls more or less like the sterile leaves, either in cauline bands or clustered in sessile or pedunculate, terminal strobili. Sporangia are solitary at the leaf base, subglobose to reniform, opening by a transverse slit; spores uniform, tetrahedral. Gametophytes epigeous, green and thalloid, or fleshy, subterranean and mycorrhizal."[2]
"Sterile stems not dorsiventral, arching and rooting at the tip or procumbent and rooting throughout, 2-5 dm long, 3-4 mm in diam.; leaves deciduous except for the evergreen apex (“bud”). Leaves linear-subulate, upcurving, strongly ciliate-denticulate. Fertile stems erect, widely spaced, rarely forking or fascinated, 10-45 cm long, 2-3 mm in diam., 5-13 mm broad including the leaves which are as those of the procumbent branches but longer, ascending, more or less flat when dry, and densely overlapping. Strobili usually solitary, 2-10 cm long, 11-20 mm broad, conspicuously broader than the stem, Sporophylls subulate, spreading, strong ciliate-denticulate. Sporangia are globose. Plants without gemmae."[2]
Distribution
This plant ranges from southeast Massachusetts to Florida, and west to eastern Texas. There are disjunct populations in the Cumberland Plateau of Kentucky, Tennessee, and Virginia, the Allegheny Mountains of West Virginia, the eastern Highland Rim of Tennessee, and Maine. There are also populations in southern Mexico through Central America to South America and Cuba.[1]
Ecology
Habitat
L. alopecuroides occurs in moist to wet semi-shaded soils.[3] Typical soil types range from sandy-peaty soils to loamy sand.[3] This species can be found in several native habitats, but also in disturbed areas. Native habitat types include wet pine savanna,[4] hillside bogs, seasonally inundated meadows, damp grassy palmetto woods, and on the edges of ponds, creeks, and titi thickets.[3] It also occurs in disturbed habitat like powerline corridors, roadsides, railways, pine plantations, and borrow pits.[3] Associated species include Ilex myrtifolia, Nyssa biflora, Pinus palutris, Aristida stricta, Cyrilla racemiflora, Juncus, Sarracenia, Rhynchospora, Xyris, Eriocaulon, Liquidambar styraciflua, Quercus laevis, Pinguicula, Kalmia hirsuta, Serenoa repens, Hypericum, Cliftonia, and Magnolia virginiana.[3]
Fire ecology
This species occurs in habitat that is maintained by frequent or annual fire.[3]
Herbivory and toxicology
Lycopodiella alopecuroides has been observed at the Archbold Biological Station to host sweat bees such as Augochlorella aurata (family Halictidae).[5]
Conservation, cultivation, and restoration
Cultural use
Photo Gallery
References and notes
- ↑ 1.0 1.1 1.2 1.3 Weakley, A.S. 2015. Flora of the southern and mid-atlantic states. Working Draft of 21 May 2015. University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
- ↑ 2.0 2.1 Radford, Albert E., Harry E. Ahles, and C. Ritchie Bell. Manual of the Vascular Flora of the Carolinas. 1964, 1968. The University of North Carolina Press. 3-6. Print.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: June 2014. Collectors: F. Almeda, Loran C. Anderson, K. Craddock Burks, A. H. Curtiss, Mary Davis, J. P. Gillespie Robert K. Godfrey, Richard D. Houk, C. Jackson, Michael R. Jenkins, Roy Komarek, R. Kral, O. Lakela, R. Lazor, S. W. Leonard, Sidney McDaniel, John T. Mickel, Leon Neel, James H. Peck, Alan R. Smith, E. Laurence Thurston, L. B. Trott, E.S. Ford, P.L. Redfearn, Jr., Allen G. Shuey, Travis MacClendon, Karen MacClendon, and Floyd Griffith. States and Counties: Florida: Alachua, Baker, Bay, Calhoun, Clay, Duval, Franklin, Gulf, Hillsborough, Jackson, Jefferson, Leon, Liberty, Manatee, Okaloosa, Santa Rosa, Wakulla, and Washington. Georgia: Grady and Thomas.
- ↑ Brewer, J. S., D. J. Baker, et al. (2011). "Carnivory in plants as a beneficial trait in wetlands." Aquatic Botany 94: 62-70.
- ↑ Deyrup, M.A. and N.D. 2015. Database of observations of Hymenoptera visitations to flowers of plants on Archbold Biological Station, Florida, USA.