Difference between revisions of "Rubus cuneifolius"
Krobertson (talk | contribs) |
ParkerRoth (talk | contribs) |
||
| (23 intermediate revisions by 10 users not shown) | |||
| Line 20: | Line 20: | ||
==Taxonomic Notes== | ==Taxonomic Notes== | ||
| − | Varieties: ''R. cuneifolius'' var. ''angustior''; ''R. cuneifolius'' var. ''subellipticus''; ''R. cuneifolius'' var. ''spiniceps''<ref name="USDA"/><br> | + | Varieties: ''R. cuneifolius'' var. ''angustior''; ''R. cuneifolius'' var. ''subellipticus''; ''R. cuneifolius'' var. ''spiniceps''<ref name="USDA"/><br>; ''R. sejunctus'' L.H. Bailey |
Synonyms: ''R. chapmannii''; ''R. dixiensis'' | Synonyms: ''R. chapmannii''; ''R. dixiensis'' | ||
| Line 26: | Line 26: | ||
==Description==<!-- Basic life history facts such as annual/perrenial, monoecious/dioecious, root morphology, seed type, etc. --> | ==Description==<!-- Basic life history facts such as annual/perrenial, monoecious/dioecious, root morphology, seed type, etc. --> | ||
''Rubus cuneifolius'' is a dioecious perennial subshrub.<ref name="USDA"/> | ''Rubus cuneifolius'' is a dioecious perennial subshrub.<ref name="USDA"/> | ||
| + | |||
| + | ''Rubus cuneifolius'' does not have specialized underground storage units apart from its taproot.<ref name="Diaz"> Diaz-Toribio, M.H. and F. E. Putz 2021. Underground carbohydrate stores and storage organs in fire-maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108: 432-442.</ref> Diaz-Toribio and Putz (2021) recorded this species to have an non-structural carbohydrate concentration of 60.3 mg/g (ranking 44 out of 100 species studied) and water content of 57.1% (ranking 59 out of 100 species studied).<ref name="Diaz"/> | ||
| + | |||
| + | According to Diaz-Torbio and Putz (2021), ''Rubus cuneifolius'' has taproots with a below-ground to above-ground biomass ratio of 11.8 and nonstructural carbohydrate concentration of 60.3 mg g<sup>-1</sup>.<ref>Diaz‐Toribio, M. H. and F. E. Putz. 2021. Underground carbohydrate stores and storage organs in fire‐maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108(3):432-442.</ref> | ||
==Distribution== | ==Distribution== | ||
| Line 32: | Line 36: | ||
==Ecology== | ==Ecology== | ||
===Habitat=== <!--Natural communities, human disturbed habitats, topography, hydrology, soils, light, fire regime requirements for removal of competition, etc.--> | ===Habitat=== <!--Natural communities, human disturbed habitats, topography, hydrology, soils, light, fire regime requirements for removal of competition, etc.--> | ||
| − | ''R. cuneifolius'' | + | |
| + | ''R. cuneifolius'' has been found in wooded floodplains, hardwood forests, turkey oak ridges, Native American mounds, and longleaf pinelands. It is also found in disturbed areas including along nature trails, along firebreaks, cut over pine flatwoods, roadsides, and dump borders.<ref name="FSU"> Florida State University Herbarium Database. URL: http://herbarium.bio.fsu.edu. Last accessed: June 2021. Collectors: Loran C. Anderson, C. H. Beck, Kathy Craddock Burks, Robert K. Godfrey, Robert Kral. States and counties: Florida: Alachua, Citrus, Franklin, Jackson, Jefferson, Liberty, and Wakulla.</ref> ''R. cuneifolius'' is an indicator species for the Clayhill Longleaf Woodlands community type as described in Carr et al. (2010).<ref>Carr, S.C., K.M. Robertson, and R.K. Peet. 2010. A vegetation classification of fire-dependent pinelands of Florida. Castanea 75:153-189.</ref> ''R. cuneifolius'' has shown regrowth in reestablished woodlands that were disturbed by agriculture in South Carolina coastal plain communities, making it a possible indicator species for post-agricultural woodlands.<ref>Brudvig, L.A., E Grman, C.W. Habeck, and J.A. Ledvina. (2013). Strong legacy of agricultural land use on soils and understory plant communities in longleaf pine woodlands. Forest Ecology and Management 310: 944-955.</ref> ''R. cuneifolius'' uses a medium amount of water, occurs in full sunlight to partial shade, and has a medium tolerance to calcium carbonate.<ref name="Ladybird"/> A study in the Great Dismal Swamp, Virginia, found ''R. cuneiolius'' was present in uncut-burned areas with a mean biomass of 1.16 g m<sup>-2</sup> yr<sup>-1</sup>.<ref>McKinley C. E. and Day, Jr. F. P. (1979). Herbaceous production in cut-burned, uncut-burned, and control areas of a ''Chamaecyparis thyoides'' (L.) BSP (Cupressaceae) stand in the Great Dismal Swamps. Bulletin of the Torrey Botanical Club 106(1):20-28.</ref> Additionally, a study exploring longleaf pine patch dynamics found ''R. cuneifolius'' to be most strongly represented within the gaps of longleaf pine forests.<ref>Mugnani et al. (2019). “Longleaf Pine Patch Dynamics Influence Ground-Layer Vegetation in Old-Growth Pine Savanna”.</ref> | ||
| + | |||
| + | Associated species: ''Pinus elliottii''.<ref name="ASU"> Arizona State University Vascular Plant Herbarium accessed using Southeastern Regional Network of Expertise and Collections (SERNEC) data portal. URL: http://sernecportal.org/portal/collections/index.php Last accessed: June 2021. Collectors: P. Genelle. States and Counties: Florida: Citrus.</ref> | ||
===Phenology===<!--Timing off flowering, fruiting, seed dispersal, and environmental triggers. Cite PanFlora website if appropriate: http://www.gilnelson.com/PanFlora/ --> | ===Phenology===<!--Timing off flowering, fruiting, seed dispersal, and environmental triggers. Cite PanFlora website if appropriate: http://www.gilnelson.com/PanFlora/ --> | ||
| − | + | ''R. cuneifolius'' has been observed to flower between March and June with peak inflorescence in April,<ref name="Weakley 2015"/><ref>Nelson, G. PanFlora: Plant data for the eastern United States with emphasis on the Southeastern Coastal Plains, Florida, and the Florida Panhandle. www.gilnelson.com/PanFlora/ Accessed: 6 DEC 2017</ref> with fruiting occurring from May through July.<ref name="Skeate 1987">Skeate S. T. (1987). Interactions between birds and fruits in a northern Florida hammock community. Ecology 68(2):297-309.</ref> ''R. cuneifolius'' produces a white conspicuous flower.<ref name="Ladybird"/> The timing of flowering has been shown to be sensitive to ozone levels where elevated levels cause earlier flowering but produce fewer large ripe fruits.<ref name="Chappelka 2002"/> Standing crops of fruit in Georgia flatwoods peaked at 114.5 g Dm<sup>-2</sup> with 52% of plots containing fruit in 5 year old plantations.<ref>Johnson A. S. and Landers J. L (1978). Fruit production in slash pine plantations in Georgia. The Journal of Wildlife Management 42(3):606-613.</ref> It typically does not flower and fruit until the second year following fire.<ref>Robertson, K.M. 2017. Personal observation at Tall Timbers Research Station, Tallahassee, Florida.</ref> | |
===Seed dispersal=== | ===Seed dispersal=== | ||
| − | Seeds of ''R. cuneifolius'' are likely primarily dispersed by | + | This species is thought to be dispersed by consumption by vertebrates.<ref> Kirkman, L. Katherine. Unpublished database of seed dispersal mode of plants found in Coastal Plain longleaf pine-grasslands of the Jones Ecological Research Center, Georgia.</ref> Seeds of ''R. cuneifolius'' are likely primarily dispersed by migrating birds and deer which consume the fruits.<ref>White D. W. and Stiles E. W. (1992). Bird dispersal of fruits of species introduced into eastern North America. Canadian Journal of Botany 70(8):1689-1696.</ref> |
===Seed bank and germination=== | ===Seed bank and germination=== | ||
| Line 44: | Line 51: | ||
===Fire ecology=== <!--Fire tolerance, fire dependence, adaptive fire responses--> | ===Fire ecology=== <!--Fire tolerance, fire dependence, adaptive fire responses--> | ||
| − | ''R. cuneifolius'' | + | ''R. cuneifolius'' readily resprouts following fire and is often found in burned areas as seedlings germinating from the seed bank,<ref name="Buckner & Landers 1979"/> but it appears to be somewhat intolerant of long-term competition with later successional fire-tolerant perennials.<ref> Robertson, K.M. 2017. Unpublished data from Ecosystem Services Project on Tall Timbers Research Station, Tallahassee, Florida.</ref> Removal of fire from old-field pineland for 44 years did not produce an increase in ''R. cuneifolius'' frequency, but rather a decrease from 80 individual in 1966 to 69 in 2013.<ref>Clewell A. F. (2014). Forest development 44 years after fire exclusion in formerly annually burned oldfield pine woodland, Florida. Castanea 79(3):147-167.</ref> Populations of ''R. cuneifolius'' have been known to persist through repeated annual burns.<ref>Robertson, K.M. Unpublished data collected from Pebble Hill Fire Plots, Pebble Hill Plantation, Thomasville, Georgia.</ref><ref>Glitzenstein, J. S., D. R. Streng, R. E. Masters, K. M. Robertson and S. M. Hermann 2012. Fire-frequency effects on vegetation in north Florida pinelands: Another look at the long-term Stoddard Fire Research Plots at Tall Timbers Research Station. Forest Ecology and Management 264: 197-209.</ref><ref>Platt, W.J., R. Carter, G. Nelson, W. Baker, S. Hermann, J. Kane, L. Anderson, M. Smith, K. Robertson. 2021. Unpublished species list of Wade Tract old-growth longleaf pine savanna, Thomasville, Georgia.</ref> |
===Pollination=== | ===Pollination=== | ||
''R. cuneifolius'' is recognized to attract a large number of native bees for pollination.<ref name="Ladybird">Plant database: ''Rubus cunifolius.'' (11 December 2017).Lady Bird Johnson Wildflower Center. URL: https://www.wildflower.org/plants/result.php?id_plant=RUCU</ref> | ''R. cuneifolius'' is recognized to attract a large number of native bees for pollination.<ref name="Ladybird">Plant database: ''Rubus cunifolius.'' (11 December 2017).Lady Bird Johnson Wildflower Center. URL: https://www.wildflower.org/plants/result.php?id_plant=RUCU</ref> | ||
| − | === | + | ===Herbivory and toxicology===<!--Common herbivores, granivory, insect hosting, poisonous chemicals, allelopathy, etc--> |
Berries produced by ''R. cuneifolius'' are highly palatable by browsing animals, composing 10-25% of the diet for many species of terrestrial birds, large mammals and small mammals. It is also an occasional source of cover for small mammals and terrestrial birds.<ref name="USDA"/> Because ''R. cuneifolius'' fruits in the late summer, it is likely an important source of energy for fall migrating bird species.<ref name="Skeate 1987"/> Besides attracting pollinators, ''R. cuneifolius'' is known for providing nesting materials/structure for native bees.<ref name="Ladybird"/> | Berries produced by ''R. cuneifolius'' are highly palatable by browsing animals, composing 10-25% of the diet for many species of terrestrial birds, large mammals and small mammals. It is also an occasional source of cover for small mammals and terrestrial birds.<ref name="USDA"/> Because ''R. cuneifolius'' fruits in the late summer, it is likely an important source of energy for fall migrating bird species.<ref name="Skeate 1987"/> Besides attracting pollinators, ''R. cuneifolius'' is known for providing nesting materials/structure for native bees.<ref name="Ladybird"/> | ||
| Line 55: | Line 62: | ||
''R. cuneifolius'' hosts several species of thripes (''Frankliniella'' spp.) which are tiny insects (1 mm or less) that feed on new plant growth producing cosmetic damage and sometimes transmitting diseases to the plant.<ref>Chellemi D. O., Funderburk J. E., and Hall D. W. (1994). Seasonal abundance of flower-inhabiting ''Frankliniella'' species (Thysanoptera: Thripidae) on wild plant species. Environmental Entomology 23(2):377-342.</ref> | ''R. cuneifolius'' hosts several species of thripes (''Frankliniella'' spp.) which are tiny insects (1 mm or less) that feed on new plant growth producing cosmetic damage and sometimes transmitting diseases to the plant.<ref>Chellemi D. O., Funderburk J. E., and Hall D. W. (1994). Seasonal abundance of flower-inhabiting ''Frankliniella'' species (Thysanoptera: Thripidae) on wild plant species. Environmental Entomology 23(2):377-342.</ref> | ||
| − | ==Conservation and | + | ==Conservation, cultivation, and restoration== |
| + | |||
| + | ==Cultural use== | ||
| + | Historically, in Appalachia, tea from the roots was used to treat cold symptoms, and the same tea or juice from the fruit was used to cure dysentery.<ref> Korchmal, Arnold & Connie. 1973. A Guide to the Medicinal Plants of the United States. The New York Times Book Company, New York.</ref> | ||
| + | |||
| + | The entire plant can be consumed in various ways, but the fruits are especially popular to eat fresh or to make jellies and drinks.<ref> Fernald, et al. 1958. Edible Plants of Eastern North America. Harper and Row Publishers, New York.</ref> | ||
| − | |||
==Photo Gallery== | ==Photo Gallery== | ||
<gallery widths=180px> | <gallery widths=180px> | ||
</gallery> | </gallery> | ||
==References and notes== | ==References and notes== | ||
Latest revision as of 14:35, 3 July 2024
| Rubus cuneifolius | |
|---|---|
Error creating thumbnail: Unable to save thumbnail to destination
| |
| Photo by Robert H. Mohlenbrock hosted at USDA NRCS Plants Database. | |
| Scientific classification | |
| Kingdom: | Plantae |
| Division: | Magnoliophyta - Flowering plants |
| Class: | Magnoliopsida - Dicots |
| Order: | Rosales |
| Family: | Rosaceae - Rose family |
| Genus: | Rubus - blackberry |
| Species: | R. cuneifolius |
| Binomial name | |
| Rubus cuneifolius Pursh | |
| |
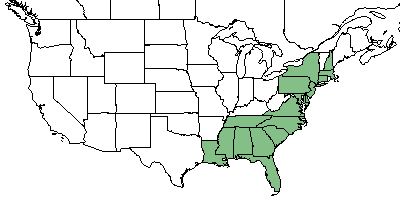
| Natural range of Rubus cuneifolius from USDA NRCS Plants Database. | |
Common Name(s): sand blackberry[1][2], sand bramble, wedge sand blackberry[2]
Contents
Taxonomic Notes
Varieties: R. cuneifolius var. angustior; R. cuneifolius var. subellipticus; R. cuneifolius var. spiniceps[2]
; R. sejunctus L.H. Bailey
Synonyms: R. chapmannii; R. dixiensis
Description
Rubus cuneifolius is a dioecious perennial subshrub.[2]
Rubus cuneifolius does not have specialized underground storage units apart from its taproot.[3] Diaz-Toribio and Putz (2021) recorded this species to have an non-structural carbohydrate concentration of 60.3 mg/g (ranking 44 out of 100 species studied) and water content of 57.1% (ranking 59 out of 100 species studied).[3]
According to Diaz-Torbio and Putz (2021), Rubus cuneifolius has taproots with a below-ground to above-ground biomass ratio of 11.8 and nonstructural carbohydrate concentration of 60.3 mg g-1.[4]
Distribution
R. cuneifolius is found primarily on the coastal plains from Connecticut and New York south to Florida, Alabama, Mississippi and Louisiana.[1][2]
Ecology
Habitat
R. cuneifolius has been found in wooded floodplains, hardwood forests, turkey oak ridges, Native American mounds, and longleaf pinelands. It is also found in disturbed areas including along nature trails, along firebreaks, cut over pine flatwoods, roadsides, and dump borders.[5] R. cuneifolius is an indicator species for the Clayhill Longleaf Woodlands community type as described in Carr et al. (2010).[6] R. cuneifolius has shown regrowth in reestablished woodlands that were disturbed by agriculture in South Carolina coastal plain communities, making it a possible indicator species for post-agricultural woodlands.[7] R. cuneifolius uses a medium amount of water, occurs in full sunlight to partial shade, and has a medium tolerance to calcium carbonate.[8] A study in the Great Dismal Swamp, Virginia, found R. cuneiolius was present in uncut-burned areas with a mean biomass of 1.16 g m-2 yr-1.[9] Additionally, a study exploring longleaf pine patch dynamics found R. cuneifolius to be most strongly represented within the gaps of longleaf pine forests.[10]
Associated species: Pinus elliottii.[11]
Phenology
R. cuneifolius has been observed to flower between March and June with peak inflorescence in April,[1][12] with fruiting occurring from May through July.[13] R. cuneifolius produces a white conspicuous flower.[8] The timing of flowering has been shown to be sensitive to ozone levels where elevated levels cause earlier flowering but produce fewer large ripe fruits.[14] Standing crops of fruit in Georgia flatwoods peaked at 114.5 g Dm-2 with 52% of plots containing fruit in 5 year old plantations.[15] It typically does not flower and fruit until the second year following fire.[16]
Seed dispersal
This species is thought to be dispersed by consumption by vertebrates.[17] Seeds of R. cuneifolius are likely primarily dispersed by migrating birds and deer which consume the fruits.[18]
Seed bank and germination
Seeds of R. cuneifolius were commonly observed in Georgia seed banks of longleaf pine - wiregrass - bracken fern forests maintained by annual spring burns since 1961.[19] Annual disking of fallow fields in different months also did not affect the presence of Rubus cuneifolius suggesting an abundance of seeds in the seed bank.[20]
Fire ecology
R. cuneifolius readily resprouts following fire and is often found in burned areas as seedlings germinating from the seed bank,[19] but it appears to be somewhat intolerant of long-term competition with later successional fire-tolerant perennials.[21] Removal of fire from old-field pineland for 44 years did not produce an increase in R. cuneifolius frequency, but rather a decrease from 80 individual in 1966 to 69 in 2013.[22] Populations of R. cuneifolius have been known to persist through repeated annual burns.[23][24][25]
Pollination
R. cuneifolius is recognized to attract a large number of native bees for pollination.[8]
Herbivory and toxicology
Berries produced by R. cuneifolius are highly palatable by browsing animals, composing 10-25% of the diet for many species of terrestrial birds, large mammals and small mammals. It is also an occasional source of cover for small mammals and terrestrial birds.[2] Because R. cuneifolius fruits in the late summer, it is likely an important source of energy for fall migrating bird species.[13] Besides attracting pollinators, R. cuneifolius is known for providing nesting materials/structure for native bees.[8]
Diseases and parasites
R. cuneifolius hosts several species of thripes (Frankliniella spp.) which are tiny insects (1 mm or less) that feed on new plant growth producing cosmetic damage and sometimes transmitting diseases to the plant.[26]
Conservation, cultivation, and restoration
Cultural use
Historically, in Appalachia, tea from the roots was used to treat cold symptoms, and the same tea or juice from the fruit was used to cure dysentery.[27]
The entire plant can be consumed in various ways, but the fruits are especially popular to eat fresh or to make jellies and drinks.[28]
Photo Gallery
References and notes
- ↑ 1.0 1.1 1.2 Weakley A. S.(2015). Flora of the Southern and Mid-Atlantic States. Chapel Hill, NC: University of North Carolina Herbarium.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 USDA, NRCS. (2016). The PLANTS Database (http://plants.usda.gov, 30 November 2017). National Plant Data Team, Greensboro, NC 27401-4901 USA.
- ↑ 3.0 3.1 Diaz-Toribio, M.H. and F. E. Putz 2021. Underground carbohydrate stores and storage organs in fire-maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108: 432-442.
- ↑ Diaz‐Toribio, M. H. and F. E. Putz. 2021. Underground carbohydrate stores and storage organs in fire‐maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108(3):432-442.
- ↑ Florida State University Herbarium Database. URL: http://herbarium.bio.fsu.edu. Last accessed: June 2021. Collectors: Loran C. Anderson, C. H. Beck, Kathy Craddock Burks, Robert K. Godfrey, Robert Kral. States and counties: Florida: Alachua, Citrus, Franklin, Jackson, Jefferson, Liberty, and Wakulla.
- ↑ Carr, S.C., K.M. Robertson, and R.K. Peet. 2010. A vegetation classification of fire-dependent pinelands of Florida. Castanea 75:153-189.
- ↑ Brudvig, L.A., E Grman, C.W. Habeck, and J.A. Ledvina. (2013). Strong legacy of agricultural land use on soils and understory plant communities in longleaf pine woodlands. Forest Ecology and Management 310: 944-955.
- ↑ 8.0 8.1 8.2 8.3 Plant database: Rubus cunifolius. (11 December 2017).Lady Bird Johnson Wildflower Center. URL: https://www.wildflower.org/plants/result.php?id_plant=RUCU
- ↑ McKinley C. E. and Day, Jr. F. P. (1979). Herbaceous production in cut-burned, uncut-burned, and control areas of a Chamaecyparis thyoides (L.) BSP (Cupressaceae) stand in the Great Dismal Swamps. Bulletin of the Torrey Botanical Club 106(1):20-28.
- ↑ Mugnani et al. (2019). “Longleaf Pine Patch Dynamics Influence Ground-Layer Vegetation in Old-Growth Pine Savanna”.
- ↑ Arizona State University Vascular Plant Herbarium accessed using Southeastern Regional Network of Expertise and Collections (SERNEC) data portal. URL: http://sernecportal.org/portal/collections/index.php Last accessed: June 2021. Collectors: P. Genelle. States and Counties: Florida: Citrus.
- ↑ Nelson, G. PanFlora: Plant data for the eastern United States with emphasis on the Southeastern Coastal Plains, Florida, and the Florida Panhandle. www.gilnelson.com/PanFlora/ Accessed: 6 DEC 2017
- ↑ 13.0 13.1 Skeate S. T. (1987). Interactions between birds and fruits in a northern Florida hammock community. Ecology 68(2):297-309.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedChappelka 2002 - ↑ Johnson A. S. and Landers J. L (1978). Fruit production in slash pine plantations in Georgia. The Journal of Wildlife Management 42(3):606-613.
- ↑ Robertson, K.M. 2017. Personal observation at Tall Timbers Research Station, Tallahassee, Florida.
- ↑ Kirkman, L. Katherine. Unpublished database of seed dispersal mode of plants found in Coastal Plain longleaf pine-grasslands of the Jones Ecological Research Center, Georgia.
- ↑ White D. W. and Stiles E. W. (1992). Bird dispersal of fruits of species introduced into eastern North America. Canadian Journal of Botany 70(8):1689-1696.
- ↑ 19.0 19.1 Buckner J. L. and Landers J. L. (1979). Fire and disking effects on herbaceous food plants and seed supplies. The Journal of Wildlife Management 43(3):807-811.
- ↑ Kay C. A. R., Clewell A. F., and Ashler E. W. (1978). Vegetative cover in a fallow field: Responses to season of soil disturbance. Bulletin of the Torrey Botanical Club 105(2):143-147.
- ↑ Robertson, K.M. 2017. Unpublished data from Ecosystem Services Project on Tall Timbers Research Station, Tallahassee, Florida.
- ↑ Clewell A. F. (2014). Forest development 44 years after fire exclusion in formerly annually burned oldfield pine woodland, Florida. Castanea 79(3):147-167.
- ↑ Robertson, K.M. Unpublished data collected from Pebble Hill Fire Plots, Pebble Hill Plantation, Thomasville, Georgia.
- ↑ Glitzenstein, J. S., D. R. Streng, R. E. Masters, K. M. Robertson and S. M. Hermann 2012. Fire-frequency effects on vegetation in north Florida pinelands: Another look at the long-term Stoddard Fire Research Plots at Tall Timbers Research Station. Forest Ecology and Management 264: 197-209.
- ↑ Platt, W.J., R. Carter, G. Nelson, W. Baker, S. Hermann, J. Kane, L. Anderson, M. Smith, K. Robertson. 2021. Unpublished species list of Wade Tract old-growth longleaf pine savanna, Thomasville, Georgia.
- ↑ Chellemi D. O., Funderburk J. E., and Hall D. W. (1994). Seasonal abundance of flower-inhabiting Frankliniella species (Thysanoptera: Thripidae) on wild plant species. Environmental Entomology 23(2):377-342.
- ↑ Korchmal, Arnold & Connie. 1973. A Guide to the Medicinal Plants of the United States. The New York Times Book Company, New York.
- ↑ Fernald, et al. 1958. Edible Plants of Eastern North America. Harper and Row Publishers, New York.