Difference between revisions of "Crotalaria rotundifolia"
Adam.Vansant (talk | contribs) |
|||
| (37 intermediate revisions by 11 users not shown) | |||
| Line 18: | Line 18: | ||
}} | }} | ||
| − | Common | + | Common names: rabbitbells, low rattlebox |
==Taxonomic notes== | ==Taxonomic notes== | ||
| − | Synonym: ''Crotalaria | + | Synonym: none<ref name=weakley>Weakley, A.S. 2020. Flora of the Southeastern United States. Edition of 20 October 2020. University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.</ref> |
| + | |||
| + | Varieties: ''Crotalaria purshii'' var. ''bracteolifera'' Fernald; ''C. purshii'' var. ''purshii''<ref name=weakley/> | ||
==Description== | ==Description== | ||
<!-- Basic life history facts such as annual/perrenial, monoecious/dioecious, root morphology, seed type, etc. --> | <!-- Basic life history facts such as annual/perrenial, monoecious/dioecious, root morphology, seed type, etc. --> | ||
| − | + | ''Crotalaria rotundifolia'' is a perennial herbaceous legume.<ref name="Davis et al 2002"/> | |
| + | |||
| + | The root system of ''Crotalaria rotundifolia'' includes stem tubers which store non-structural carbohydrates (NSC) important for both resprouting following fire and persisting during long periods of fire exclusion.<ref name="Diaz"> Diaz-Toribio, M.H. and F. E. Putz 2021. Underground carbohydrate stores and storage organs in fire-maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108: 432-442.</ref> Diaz-Toribio and Putz (2021) recorded this species to have an NSC concentration of 260.7 mg/g (ranking 15 out of 100 species studied) and water content of 55.9% (ranking 9 out of 100 species studied).<ref name = "Diaz"/> | ||
| + | |||
| + | According to Diaz-Torbio and Putz (2021), ''Crotalaria rotundifolia'' has stem tubers with a below-ground to above-ground biomass ratio of 1.46 and nonstructural carbohydrate concentration of 260.7 mg g<sup>-1</sup>.<ref>Diaz‐Toribio, M. H. and F. E. Putz. 2021. Underground carbohydrate stores and storage organs in fire‐maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108(3):432-442.</ref> | ||
==Distribution== | ==Distribution== | ||
| − | + | ''Crotalaria rotundifolia'' is found across the southern and eastern United States west to Texas and south to Monroe County, Florida, the West Indies, Mexico, and Central America.<ref name=regional>[[http://regionalconservation.org/beta/nfyn/plantdetail.asp?tx=Crotrotu]] Regional Conservation. Accessed: April 15, 2016</ref> | |
==Ecology== | ==Ecology== | ||
It is a nitrogen-fixing legume.<ref>Runion, G. B., M. A. Davis, et al. (2006). "Effects of elevated atmospheric carbon dioxide on biomass and carbon accumulation in a model regenerating longleaf pine community." Journal of Environmental Quality 35: 1478-1486.</ref> In a study by Davis, it was discovered that ''C. rotundifolia'' had higher mortality and less biomass in high carbon dioxide plots, suggesting that not all species will perform well as global carbon dioxide levels rise.<ref name="Davis et al 2002"/> | It is a nitrogen-fixing legume.<ref>Runion, G. B., M. A. Davis, et al. (2006). "Effects of elevated atmospheric carbon dioxide on biomass and carbon accumulation in a model regenerating longleaf pine community." Journal of Environmental Quality 35: 1478-1486.</ref> In a study by Davis, it was discovered that ''C. rotundifolia'' had higher mortality and less biomass in high carbon dioxide plots, suggesting that not all species will perform well as global carbon dioxide levels rise.<ref name="Davis et al 2002"/> | ||
===Habitat=== <!--Natural communities, human disturbed habitats, topography, hydrology, soils, light, fire regime requirements for removal of competition, etc.--> | ===Habitat=== <!--Natural communities, human disturbed habitats, topography, hydrology, soils, light, fire regime requirements for removal of competition, etc.--> | ||
| − | This species is a common associate in longleaf pine savannas.<ref name="Davis et al 2002">Davis, M. A., S. G. Pritchard, et al. (2002). "Elevated atmospheric CO2 affects structure of a model regenerating longleaf pine community." Journal of Ecology 90: 130-140.</ref> It can also be found in sandhill communities,<ref name="Stamp and Lucas 1990"/> oak-palmetto scrub, evergreen-scrub oak sand ridges, slash pine flatwoods, sand dunes, backdunes, dune swales, and bordering cypress swamps and depression marshes<ref name=fsu>Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: June 2014. Collectors: O. Lakela, Loran C. Anderson, Bian Tan, Brenda Herring, Don Herring, Gwynn W. Ramsey, H. Larry Stripling, R.K. Godfrey, John Morrill, R. Kral, C. Jackson, D. B. Ward, A. F. Clewell, J. Beckner, D. Burch, L B Trott, William Reese, Paul Redfearn, H. E. Grelen, R. C. Phillips, L. J. Brass, Ann F. Johnson, J. Sincock, Grady W. Reinert, Mabel Kral, Elmer C. Prichard, Sidney McDaniel, Roomie Wilson, K. Craddock Burks, W. W. Baker, A. Mellon, Richard S. Mitchell, Steve L. Orzell, Edwin L. Bridges, Patricia Elliot, A. H. Curtiss, Kurt E. Blum, Dave Breil, H. A. Lang, R. F. Doren, R. A. Norris, Walter Kittredge, R. Komarek, Chris Cooksey, Kevin Oakes, M. Davis, Cecil R Slaughter, John B. Nelson, Cynthia Aulbach-Smith, Kelley, Batson, S. M. Tracy, D. P. Bain, R. B. Carr, R. L. Wilbur, J A Duke, H. L. Blomquist, William B. Fox, A. E. Radford, H. R. Reed, Barbara Lund, Grelen, and Wilbur H Duncan. States and Counties: Florida: Bay, Brevard, Calhoun, Citrus, Clay, Collier, Columbia, Dixie, Duval, Flagler, Franklin, Gadsden, Hamilton, Hernando, Highlands, Indian River, Jackson, Jefferson, Lake, Lee, Leon, Levy, Liberty, Madison, Marion, Martin, Monroe, Nassau, Osceola, Orange, Polk, Putnam, Taylor, Santa Rosa, Sarasota, St John’s, Sumter, Suwannee, Volusia, and Wakulla. Georgia: Charlton, Echols, Grady, Lanier, and Thomas. South Carolina: Barnwell, Berkeley, Jasper, and Richland. Mississippi: George, Harrison, Lamar, and Pearl River. Texas: Brazos. North Carolina: Bladen, Carteret, and Wayne. Alabama: Baldwin and Mobile.</ref> | + | This species is a common associate in longleaf pine savannas.<ref name="Davis et al 2002">Davis, M. A., S. G. Pritchard, et al. (2002). "Elevated atmospheric CO2 affects structure of a model regenerating longleaf pine community." Journal of Ecology 90: 130-140.</ref> It can also be found in sandhill communities,<ref name="Stamp and Lucas 1990"/> oak-palmetto scrub, evergreen-scrub oak sand ridges, slash pine flatwoods, sand dunes, backdunes, dune swales, and bordering cypress swamps and depression marshes.<ref name=fsu>Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: June 2014. Collectors: O. Lakela, Loran C. Anderson, Bian Tan, Brenda Herring, Don Herring, Gwynn W. Ramsey, H. Larry Stripling, R.K. Godfrey, John Morrill, R. Kral, C. Jackson, D. B. Ward, A. F. Clewell, J. Beckner, D. Burch, L B Trott, William Reese, Paul Redfearn, H. E. Grelen, R. C. Phillips, L. J. Brass, Ann F. Johnson, J. Sincock, Grady W. Reinert, Mabel Kral, Elmer C. Prichard, Sidney McDaniel, Roomie Wilson, K. Craddock Burks, W. W. Baker, A. Mellon, Richard S. Mitchell, Steve L. Orzell, Edwin L. Bridges, Patricia Elliot, A. H. Curtiss, Kurt E. Blum, Dave Breil, H. A. Lang, R. F. Doren, R. A. Norris, Walter Kittredge, R. Komarek, Chris Cooksey, Kevin Oakes, M. Davis, Cecil R Slaughter, John B. Nelson, Cynthia Aulbach-Smith, Kelley, Batson, S. M. Tracy, D. P. Bain, R. B. Carr, R. L. Wilbur, J A Duke, H. L. Blomquist, William B. Fox, A. E. Radford, H. R. Reed, Barbara Lund, Grelen, and Wilbur H Duncan. States and Counties: Florida: Bay, Brevard, Calhoun, Citrus, Clay, Collier, Columbia, Dixie, Duval, Flagler, Franklin, Gadsden, Hamilton, Hernando, Highlands, Indian River, Jackson, Jefferson, Lake, Lee, Leon, Levy, Liberty, Madison, Marion, Martin, Monroe, Nassau, Osceola, Orange, Polk, Putnam, Taylor, Santa Rosa, Sarasota, St John’s, Sumter, Suwannee, Volusia, and Wakulla. Georgia: Charlton, Echols, Grady, Lanier, and Thomas. South Carolina: Barnwell, Berkeley, Jasper, and Richland. Mississippi: George, Harrison, Lamar, and Pearl River. Texas: Brazos. North Carolina: Bladen, Carteret, and Wayne. Alabama: Baldwin and Mobile.</ref> This species also occurs in disturbed areas including roadsides, clearings in turkey oak barrens, and clear-cut flatwoods. ''C. rotundifolia'' prefers higher light levels associated with open pinelands, and sandy soil types, both moist and dry, such as coarse sand, drying sand, peaty sand, and sandy clay.<ref name=fsu/> However, ''C. rotundifolia'' was found to be neutral in its short-term response to single mechanical soil disturbances as well as in its long-term response following cessation of repeated soil disturbance.<ref name=Dixon>Dixon, C. M., K. M. Robertson, A. M. Reid and M. T. Rother. 2024. Mechanical soil disturbance in a pine savanna has multiyear effects on plant species composition. Ecosphere 15(2):e4759.</ref> |
| − | '' | + | Associated species includes ''Rhynchospora, Xyris,'' Longleaf pine, wiregrass, oak, saw palmetto, ''Quercus laevis, Q. margaretta, Aristida beyrichiana'', slash pine, ''Polygala nana, Cyperus lecontei, Polypremum procumbens, Crotonopsis, Paronychia, Andropogon, Diospyros, Aristida, Cnidoscolus. Eupoatorium compositifolium, Axonopus affinis'' and others.<ref name=fsu/> |
| − | + | ''Crotalaria rotundifolia'' is frequent and abundant in the Peninsula Xeric Sandhills and North Florida Subxeric Sandhills community types as described in Carr et al. (2010).<ref>Carr, S.C., K.M. Robertson, and R.K. Peet. 2010. A vegetation classification of fire-dependent pinelands of Florida. Castanea 75:153-189.</ref> | |
===Phenology=== <!--Timing off flowering, fruiting, seed dispersal, and environmental triggers. Cite PanFlora website if appropriate: http://www.gilnelson.com/PanFlora/ --> | ===Phenology=== <!--Timing off flowering, fruiting, seed dispersal, and environmental triggers. Cite PanFlora website if appropriate: http://www.gilnelson.com/PanFlora/ --> | ||
| − | It has a broad, bimodal flowering phenology with peaks in early April and late fall.<ref name="Hiers et al 2000">Hiers, J. K., R. Wyatt, et al. (2000). "The effects of fire regime on legume reproduction in longleaf pine savannas: is a season selective?" Oecologia 125: 521-530.</ref> Flowering has been observed in March through December, | + | It has a broad, bimodal flowering phenology with peaks in early April and late fall.<ref name="Hiers et al 2000">Hiers, J. K., R. Wyatt, et al. (2000). "The effects of fire regime on legume reproduction in longleaf pine savannas: is a season selective?" Oecologia 125: 521-530.</ref> Flowering has been observed in March through December with peak inflorescence in April.<ref>Nelson, G. PanFlora: Plant data for the eastern United States with emphasis on the Southeastern Coastal Plains, Florida, and the Florida Panhandle. www.gilnelson.com/PanFlora/ Accessed: 22 APR 2019</ref> Fruiting has been observed in April through November. <ref name=fsu/> However, in its southern natural regions, ''C. rotundifolia'' can flower year-round.<ref name= "lady bird">[[https://www.wildflower.org/plants/search.php?search_field=&newsearch=true]] Lady Bird Johnson Wildflower Center. Accessed: April 22, 2019</ref> |
===Seed dispersal=== | ===Seed dispersal=== | ||
| − | Seeds are forcefully expelled after the fruit matures and dries, and ants act as the main dispersal agents. The ballistic dispersal distance was found to be around .94 meters.<ref name="Stamp and Lucas 1990">Stamp, N. E. and J. R. Lucas (1990). "Spatial patterns and dispersal distances of explosively dispersing plants in Florida sandhill vegetation." Journal of Ecology 78: 589-600.</ref> | + | Seeds are forcefully expelled after the fruit matures and dries, and ants act as the main dispersal agents. The ballistic dispersal distance was found to be around .94 meters.<ref name="Stamp and Lucas 1990">Stamp, N. E. and J. R. Lucas (1990). "Spatial patterns and dispersal distances of explosively dispersing plants in Florida sandhill vegetation." Journal of Ecology 78: 589-600.</ref> This species is thought to be dispersed by ants and/or explosive dehiscence. <ref> Kirkman, L. Katherine. Unpublished database of seed dispersal mode of plants found in Coastal Plain longleaf pine-grasslands of the Jones Ecological Research Center, Georgia.</ref> |
| − | + | ||
| + | ===Seed bank and germination=== | ||
| + | A study by Scott Wiggers found seeds of ''C. rotundifolia'' to need 9 seconds of scarification for successful germination.<ref name= "Wiggers"/> This is due to the fact that these are particularly hard-seeded.<ref name= "Wiggers2">Wiggers, M. S., et al. (2013). "Fine-scale variation in surface fire environment and legume germination in the longleaf pine ecosystem." Forest Ecology and Management 310: 54-63.</ref> They also found more success of germination if the seeds were exposed to some level of dry heat as well as with steam heat exposure.<ref name= "Wiggers">Wiggers, M. S., et al. (2017). "Seed heat tolerance and germination of six legume species native to a fire-prone longleaf pine forest." Plant Ecology 218: 151-171.</ref> Another study by Wiggers found that germination was greater with a lower mortality post-burn with low amounts of fine fuel rather than with high amounts of fine fuels.<ref name= "Wiggers2"/> | ||
===Fire ecology=== <!--Fire tolerance, fire dependence, adaptive fire responses--> | ===Fire ecology=== <!--Fire tolerance, fire dependence, adaptive fire responses--> | ||
| − | Robustness of reproduction is related to burn treatments and season of burn.<ref name="Hiers et al 2000"/> | + | Robustness of reproduction is related to burn treatments and season of burn.<ref name="Hiers et al 2000"/> A study found that ''Crotalaria rotundifolia'' produced the greatest number of flowers after a lightning season burn (19.5) than after an instance of no fire (19.1) or after a late winter/early spring burn (17.2).<ref>Hiers, J. K., et al. (2000). "The effects of fire regime on legume reproduction in longleaf pine savannas: is a season selective?" Oecologia 125: 521-530.</ref> This study also found that the duration of synchronous flowering was the same after a lightning season burn (210.7 days) and after instances of no fire (210.7 days) than compared to after a late winter/early spring burn (190.0 days).<ref>Hiers, J. K., et al. (2000). "The effects of fire regime on legume reproduction in longleaf pine savannas: is a season selective?" Oecologia 125: 521-530.</ref> Additionally, peak flowering activity occurred at the same time after a lightning season burn (190.0 Julian) and after an instance of no fire (190.0 Julian) than compared to after a late winter/early spring burn (205.7 Julian).<ref>Hiers, J. K., et al. (2000). "The effects of fire regime on legume reproduction in longleaf pine savannas: is a season selective?" Oecologia 125: 521-530.</ref> Seedling emergence has been shown to significantly increase over a year for a burned site rather than an unburned site.<ref>Parks, G. R. (2007). Longleaf pine sandhill seed banks and seedling emergence in relation to time since fire, University of Florida. Master of Science: 84.</ref> However, one study found germination to be unaffected by fire.<ref name= "Wiggers2"/> |
| + | |||
| + | Populations of ''c. rotundifolia'' have been known to persist through repeated annual burns.<ref>Robertson, K.M. Unpublished data collected from Pebble Hill Fire Plots, Pebble Hill Plantation, Thomasville, Georgia.</ref><ref>Glitzenstein, J. S., D. R. Streng, R. E. Masters, K. M. Robertson and S. M. Hermann 2012. Fire-frequency effects on vegetation in north Florida pinelands: Another look at the long-term Stoddard Fire Research Plots at Tall Timbers Research Station. Forest Ecology and Management 264: 197-209.</ref> | ||
===Pollination=== | ===Pollination=== | ||
| − | + | ''Crotalaria rotundifolia'' was observed at the Archbold Biological Station to host leafcutting bees from the Megachilidae family such as ''Megachile brevis pseudobrevis''.<ref name=dey> Deyrup, M.A. and N.D. 2015. Database of observations of Hymenoptera visitations to flowers of plants on Archbold Biological Station, Florida, USA. </ref> | |
| − | + | ===Herbivory and toxicology=== | |
| + | Caterpillars are often found consuming ''C. rotundifolia''. Ants, especially ''Pogonomyrmex badius,'' help disperse the seeds long distances.<ref name="Stamp and Lucas 1990"/> It is the larval host plant for the ceranus blue (''Hemiargus ceraunus'') butterfly.<ref name=regional/> | ||
| − | === | + | ===Diseases and parasites=== |
| − | + | ''C. rotundifolia'' can be infected by root-knot nematodes, including ''Meloidogyne arenaria'' and ''M. javanica''.<ref>Quesenberry, K. H., et al. (2008). "Response of native southeastern U.S. legumes to root-knot nematodes." Crop Science 48: 2274-2278.</ref> | |
| − | + | ==Conservation, cultivation, and restoration== | |
| − | + | This species is listed as endangered by the Maryland Department of Natural Resources. As well, the genus ''Crotalaria'' is listed as a noxious weed by the Arkansas State Plant Board.<ref name= "USDA">USDA, NRCS. (2016). The PLANTS Database (http://plants.usda.gov, 22 April 2019). National Plant Data Team, Greensboro, NC 27401-4901 USA.</ref> | |
| + | ==Cultural use== | ||
| + | The seeds may be used as a substitute for coffee, but it is not recommended as improper preparation could result in poisoning.<ref> Fernald, et al. 1958. Edible Plants of Eastern North America. Harper and Row Publishers, New York.</ref> | ||
| − | |||
| − | |||
==Photo Gallery== | ==Photo Gallery== | ||
<gallery widths=180px> | <gallery widths=180px> | ||
Latest revision as of 16:51, 1 August 2024
| Crotalaria rotundifolia | |
|---|---|

| |
| Photo was taken by Gil Nelson | |
| Scientific classification | |
| Kingdom: | Plantae |
| Division: | Magnoliophyta - Flowering plants |
| Class: | Magnoliopsida – Dicotyledons |
| Order: | Fabales |
| Family: | Fabaceae ⁄ Leguminosae |
| Genus: | Crotalaria |
| Species: | C. rotundifolia |
| Binomial name | |
| Crotalaria rotundifolia Walter ex J.F. Gmel. | |
| |
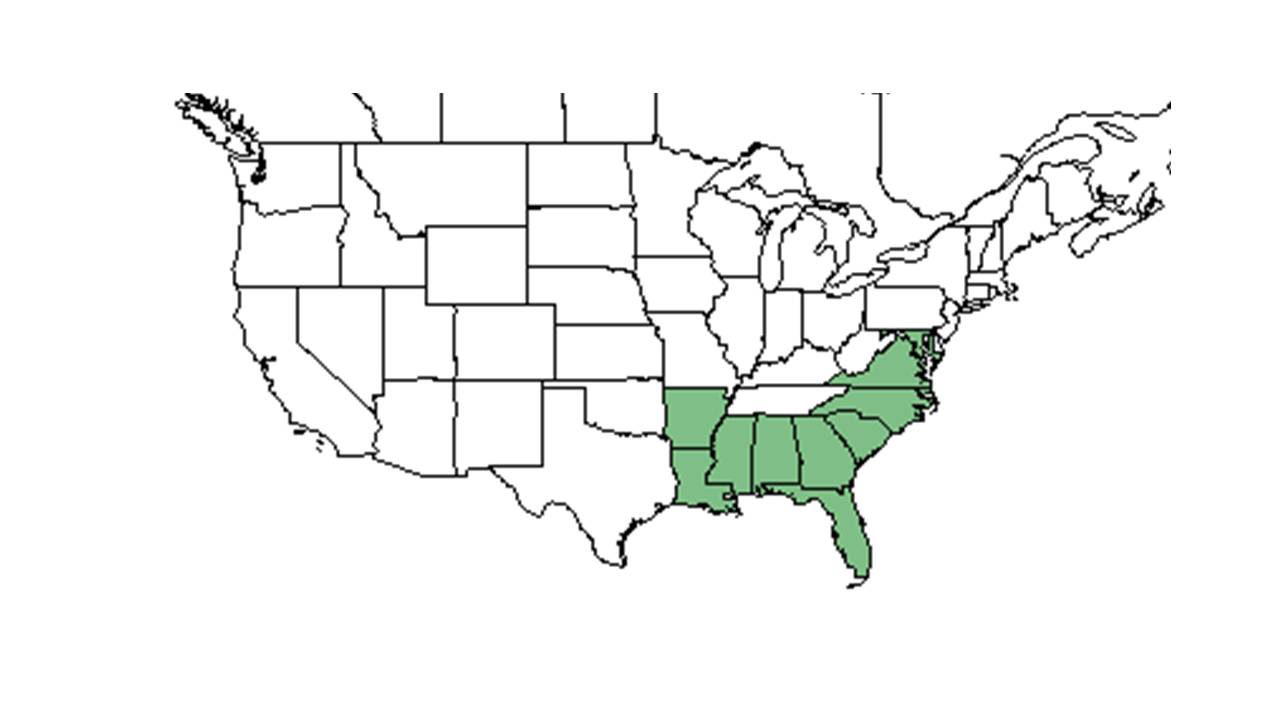
| Natural range of Crotalaria rotundifolia from USDA NRCS Plants Database. | |
Common names: rabbitbells, low rattlebox
Contents
Taxonomic notes
Synonym: none[1]
Varieties: Crotalaria purshii var. bracteolifera Fernald; C. purshii var. purshii[1]
Description
Crotalaria rotundifolia is a perennial herbaceous legume.[2]
The root system of Crotalaria rotundifolia includes stem tubers which store non-structural carbohydrates (NSC) important for both resprouting following fire and persisting during long periods of fire exclusion.[3] Diaz-Toribio and Putz (2021) recorded this species to have an NSC concentration of 260.7 mg/g (ranking 15 out of 100 species studied) and water content of 55.9% (ranking 9 out of 100 species studied).[3]
According to Diaz-Torbio and Putz (2021), Crotalaria rotundifolia has stem tubers with a below-ground to above-ground biomass ratio of 1.46 and nonstructural carbohydrate concentration of 260.7 mg g-1.[4]
Distribution
Crotalaria rotundifolia is found across the southern and eastern United States west to Texas and south to Monroe County, Florida, the West Indies, Mexico, and Central America.[5]
Ecology
It is a nitrogen-fixing legume.[6] In a study by Davis, it was discovered that C. rotundifolia had higher mortality and less biomass in high carbon dioxide plots, suggesting that not all species will perform well as global carbon dioxide levels rise.[2]
Habitat
This species is a common associate in longleaf pine savannas.[2] It can also be found in sandhill communities,[7] oak-palmetto scrub, evergreen-scrub oak sand ridges, slash pine flatwoods, sand dunes, backdunes, dune swales, and bordering cypress swamps and depression marshes.[8] This species also occurs in disturbed areas including roadsides, clearings in turkey oak barrens, and clear-cut flatwoods. C. rotundifolia prefers higher light levels associated with open pinelands, and sandy soil types, both moist and dry, such as coarse sand, drying sand, peaty sand, and sandy clay.[8] However, C. rotundifolia was found to be neutral in its short-term response to single mechanical soil disturbances as well as in its long-term response following cessation of repeated soil disturbance.[9]
Associated species includes Rhynchospora, Xyris, Longleaf pine, wiregrass, oak, saw palmetto, Quercus laevis, Q. margaretta, Aristida beyrichiana, slash pine, Polygala nana, Cyperus lecontei, Polypremum procumbens, Crotonopsis, Paronychia, Andropogon, Diospyros, Aristida, Cnidoscolus. Eupoatorium compositifolium, Axonopus affinis and others.[8]
Crotalaria rotundifolia is frequent and abundant in the Peninsula Xeric Sandhills and North Florida Subxeric Sandhills community types as described in Carr et al. (2010).[10]
Phenology
It has a broad, bimodal flowering phenology with peaks in early April and late fall.[11] Flowering has been observed in March through December with peak inflorescence in April.[12] Fruiting has been observed in April through November. [8] However, in its southern natural regions, C. rotundifolia can flower year-round.[13]
Seed dispersal
Seeds are forcefully expelled after the fruit matures and dries, and ants act as the main dispersal agents. The ballistic dispersal distance was found to be around .94 meters.[7] This species is thought to be dispersed by ants and/or explosive dehiscence. [14]
Seed bank and germination
A study by Scott Wiggers found seeds of C. rotundifolia to need 9 seconds of scarification for successful germination.[15] This is due to the fact that these are particularly hard-seeded.[16] They also found more success of germination if the seeds were exposed to some level of dry heat as well as with steam heat exposure.[15] Another study by Wiggers found that germination was greater with a lower mortality post-burn with low amounts of fine fuel rather than with high amounts of fine fuels.[16]
Fire ecology
Robustness of reproduction is related to burn treatments and season of burn.[11] A study found that Crotalaria rotundifolia produced the greatest number of flowers after a lightning season burn (19.5) than after an instance of no fire (19.1) or after a late winter/early spring burn (17.2).[17] This study also found that the duration of synchronous flowering was the same after a lightning season burn (210.7 days) and after instances of no fire (210.7 days) than compared to after a late winter/early spring burn (190.0 days).[18] Additionally, peak flowering activity occurred at the same time after a lightning season burn (190.0 Julian) and after an instance of no fire (190.0 Julian) than compared to after a late winter/early spring burn (205.7 Julian).[19] Seedling emergence has been shown to significantly increase over a year for a burned site rather than an unburned site.[20] However, one study found germination to be unaffected by fire.[16]
Populations of c. rotundifolia have been known to persist through repeated annual burns.[21][22]
Pollination
Crotalaria rotundifolia was observed at the Archbold Biological Station to host leafcutting bees from the Megachilidae family such as Megachile brevis pseudobrevis.[23]
Herbivory and toxicology
Caterpillars are often found consuming C. rotundifolia. Ants, especially Pogonomyrmex badius, help disperse the seeds long distances.[7] It is the larval host plant for the ceranus blue (Hemiargus ceraunus) butterfly.[5]
Diseases and parasites
C. rotundifolia can be infected by root-knot nematodes, including Meloidogyne arenaria and M. javanica.[24]
Conservation, cultivation, and restoration
This species is listed as endangered by the Maryland Department of Natural Resources. As well, the genus Crotalaria is listed as a noxious weed by the Arkansas State Plant Board.[25]
Cultural use
The seeds may be used as a substitute for coffee, but it is not recommended as improper preparation could result in poisoning.[26]
Photo Gallery
References and notes
- ↑ 1.0 1.1 Weakley, A.S. 2020. Flora of the Southeastern United States. Edition of 20 October 2020. University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
- ↑ 2.0 2.1 2.2 Davis, M. A., S. G. Pritchard, et al. (2002). "Elevated atmospheric CO2 affects structure of a model regenerating longleaf pine community." Journal of Ecology 90: 130-140.
- ↑ 3.0 3.1 Diaz-Toribio, M.H. and F. E. Putz 2021. Underground carbohydrate stores and storage organs in fire-maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108: 432-442.
- ↑ Diaz‐Toribio, M. H. and F. E. Putz. 2021. Underground carbohydrate stores and storage organs in fire‐maintained longleaf pine savannas in Florida, USA. American Journal of Botany 108(3):432-442.
- ↑ 5.0 5.1 [[1]] Regional Conservation. Accessed: April 15, 2016
- ↑ Runion, G. B., M. A. Davis, et al. (2006). "Effects of elevated atmospheric carbon dioxide on biomass and carbon accumulation in a model regenerating longleaf pine community." Journal of Environmental Quality 35: 1478-1486.
- ↑ 7.0 7.1 7.2 Stamp, N. E. and J. R. Lucas (1990). "Spatial patterns and dispersal distances of explosively dispersing plants in Florida sandhill vegetation." Journal of Ecology 78: 589-600.
- ↑ 8.0 8.1 8.2 8.3 Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: June 2014. Collectors: O. Lakela, Loran C. Anderson, Bian Tan, Brenda Herring, Don Herring, Gwynn W. Ramsey, H. Larry Stripling, R.K. Godfrey, John Morrill, R. Kral, C. Jackson, D. B. Ward, A. F. Clewell, J. Beckner, D. Burch, L B Trott, William Reese, Paul Redfearn, H. E. Grelen, R. C. Phillips, L. J. Brass, Ann F. Johnson, J. Sincock, Grady W. Reinert, Mabel Kral, Elmer C. Prichard, Sidney McDaniel, Roomie Wilson, K. Craddock Burks, W. W. Baker, A. Mellon, Richard S. Mitchell, Steve L. Orzell, Edwin L. Bridges, Patricia Elliot, A. H. Curtiss, Kurt E. Blum, Dave Breil, H. A. Lang, R. F. Doren, R. A. Norris, Walter Kittredge, R. Komarek, Chris Cooksey, Kevin Oakes, M. Davis, Cecil R Slaughter, John B. Nelson, Cynthia Aulbach-Smith, Kelley, Batson, S. M. Tracy, D. P. Bain, R. B. Carr, R. L. Wilbur, J A Duke, H. L. Blomquist, William B. Fox, A. E. Radford, H. R. Reed, Barbara Lund, Grelen, and Wilbur H Duncan. States and Counties: Florida: Bay, Brevard, Calhoun, Citrus, Clay, Collier, Columbia, Dixie, Duval, Flagler, Franklin, Gadsden, Hamilton, Hernando, Highlands, Indian River, Jackson, Jefferson, Lake, Lee, Leon, Levy, Liberty, Madison, Marion, Martin, Monroe, Nassau, Osceola, Orange, Polk, Putnam, Taylor, Santa Rosa, Sarasota, St John’s, Sumter, Suwannee, Volusia, and Wakulla. Georgia: Charlton, Echols, Grady, Lanier, and Thomas. South Carolina: Barnwell, Berkeley, Jasper, and Richland. Mississippi: George, Harrison, Lamar, and Pearl River. Texas: Brazos. North Carolina: Bladen, Carteret, and Wayne. Alabama: Baldwin and Mobile.
- ↑ Dixon, C. M., K. M. Robertson, A. M. Reid and M. T. Rother. 2024. Mechanical soil disturbance in a pine savanna has multiyear effects on plant species composition. Ecosphere 15(2):e4759.
- ↑ Carr, S.C., K.M. Robertson, and R.K. Peet. 2010. A vegetation classification of fire-dependent pinelands of Florida. Castanea 75:153-189.
- ↑ 11.0 11.1 Hiers, J. K., R. Wyatt, et al. (2000). "The effects of fire regime on legume reproduction in longleaf pine savannas: is a season selective?" Oecologia 125: 521-530.
- ↑ Nelson, G. PanFlora: Plant data for the eastern United States with emphasis on the Southeastern Coastal Plains, Florida, and the Florida Panhandle. www.gilnelson.com/PanFlora/ Accessed: 22 APR 2019
- ↑ [[2]] Lady Bird Johnson Wildflower Center. Accessed: April 22, 2019
- ↑ Kirkman, L. Katherine. Unpublished database of seed dispersal mode of plants found in Coastal Plain longleaf pine-grasslands of the Jones Ecological Research Center, Georgia.
- ↑ 15.0 15.1 Wiggers, M. S., et al. (2017). "Seed heat tolerance and germination of six legume species native to a fire-prone longleaf pine forest." Plant Ecology 218: 151-171.
- ↑ 16.0 16.1 16.2 Wiggers, M. S., et al. (2013). "Fine-scale variation in surface fire environment and legume germination in the longleaf pine ecosystem." Forest Ecology and Management 310: 54-63.
- ↑ Hiers, J. K., et al. (2000). "The effects of fire regime on legume reproduction in longleaf pine savannas: is a season selective?" Oecologia 125: 521-530.
- ↑ Hiers, J. K., et al. (2000). "The effects of fire regime on legume reproduction in longleaf pine savannas: is a season selective?" Oecologia 125: 521-530.
- ↑ Hiers, J. K., et al. (2000). "The effects of fire regime on legume reproduction in longleaf pine savannas: is a season selective?" Oecologia 125: 521-530.
- ↑ Parks, G. R. (2007). Longleaf pine sandhill seed banks and seedling emergence in relation to time since fire, University of Florida. Master of Science: 84.
- ↑ Robertson, K.M. Unpublished data collected from Pebble Hill Fire Plots, Pebble Hill Plantation, Thomasville, Georgia.
- ↑ Glitzenstein, J. S., D. R. Streng, R. E. Masters, K. M. Robertson and S. M. Hermann 2012. Fire-frequency effects on vegetation in north Florida pinelands: Another look at the long-term Stoddard Fire Research Plots at Tall Timbers Research Station. Forest Ecology and Management 264: 197-209.
- ↑ Deyrup, M.A. and N.D. 2015. Database of observations of Hymenoptera visitations to flowers of plants on Archbold Biological Station, Florida, USA.
- ↑ Quesenberry, K. H., et al. (2008). "Response of native southeastern U.S. legumes to root-knot nematodes." Crop Science 48: 2274-2278.
- ↑ USDA, NRCS. (2016). The PLANTS Database (http://plants.usda.gov, 22 April 2019). National Plant Data Team, Greensboro, NC 27401-4901 USA.
- ↑ Fernald, et al. 1958. Edible Plants of Eastern North America. Harper and Row Publishers, New York.