Difference between revisions of "Heterotheca subaxillaris"
Emmazeitler (talk | contribs) (→Distribution) |
HaleighJoM (talk | contribs) (→Ecology) |
||
| (6 intermediate revisions by 3 users not shown) | |||
| Line 48: | Line 48: | ||
===Pollination=== | ===Pollination=== | ||
| − | + | ''Heterotheca subaxillaris'' has been observed at the Archbold Biological Station to be pollinated by ground-nesting bees from the Andrenidae family (''Andrena fulvipennis''), sweat wasps from the Halictidae family (''Agapostemon splendens, Augochloropsis metallica, A. sumptuosa, Halictus poeyi, Lasioglossum nymphalis'' and ''L. tamiamensis''), leafcutting bees from the Megachilidae family (''Anthidium maculifrons, Coelioxys mexicana, Megachile albitarsis, M. brevis pseudobrevis, M. inimica'' and ''M. xylocopoides''), thread-waisted wasps from the Sphecidae family (''Bicyrtes capnoptera, Microbembex monodonta'' and ''Tachytes validus''), and wasps from the Vespidae family (''Pachodynerus erynnis, Stenodynerus fundatiformis'' and ''Zethus spinipes'').<ref name="Deyrup 2015">Deyrup, M.A. and N.D. 2015. Database of observations of Hymenoptera visitations to flowers of plants on Archbold Biological Station, Florida, USA.</ref> Additionally, this species has been observed with ground-nesting bees from the Andrenidae family (''Calliopsis sp., Macrotera sp., Perdita sp., Protandrena sp.,'' and ''Pseudopanurgus sp.''), long-tongued bees from the Apidae family (''Anthophora sp., Bombus sp., Ceratina sp., Exomalopsis sp., Holcopasites sp., Nomada sp., Svastra sp.'' and ''Tetraloniella sp.''), plasterer bees from the Colletidae family (''Colletes sp.''), sweat bees from the Halictidae family (''Agapostemon sp., Dieunomia sp., Halictus sp.'' and ''Lasioglossum sp.''), leafcutting bees from the Megachille family (''Ashmeadiella sp., Coelioxys sp., Dianthidium sp., Heriades sp., Megachile sp.'' and ''Paranthidium sp.''), thread-waisted wasps from the Sphecidae family (''Sphex ichneumoneus''), planthoppers from the Delphacidae family (''Pissonotus delicatus''), plant bugs from the Miridae family (''Macrotylus amoenus''), stink bugs from the Pentatomidae family (''Euschistus servus''), and assassin bugs from the Reduviidae family (''Apiomerus crassipes'' and ''Apiomerus flaviventris'').<ref>Discoverlife.org [https://www.discoverlife.org/20/q?search=Bidens+albaDiscoverlife.org|Discoverlife.org]</ref> | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
In defoliated plants, there is an increased biomass allocation to shoot growth, maintaining a balance between carbon and nutrient income similar to non defoliated plants. Defoliated plants have a slow growth rate, suggesting that even though proportionally greater allocation to the shoots occurs after defoliation, both carbon income and nutrient uptake are reduced. <ref name="Mihaliak and Lincoln 1989"/> | In defoliated plants, there is an increased biomass allocation to shoot growth, maintaining a balance between carbon and nutrient income similar to non defoliated plants. Defoliated plants have a slow growth rate, suggesting that even though proportionally greater allocation to the shoots occurs after defoliation, both carbon income and nutrient uptake are reduced. <ref name="Mihaliak and Lincoln 1989"/> | ||
| + | <!--===Herbivory and toxicology===--> | ||
<!--===Diseases and parasites===--> | <!--===Diseases and parasites===--> | ||
| − | |||
| − | == | + | ==Conservation, cultivation, and restoration== |
| + | |||
| + | ==Cultural use== | ||
==Photo Gallery== | ==Photo Gallery== | ||
<gallery widths=180px> | <gallery widths=180px> | ||
Latest revision as of 20:24, 30 June 2022
| Heterotheca subaxillaris | |
|---|---|

| |
| Photo by Allen Boatman, Atlas of Florida Vascular Plants | |
| Scientific classification | |
| Kingdom: | Plantae |
| Division: | Magnoliophyta - Flowering plants |
| Class: | Magnoliopsida - Dicotyledons |
| Order: | Asterales |
| Family: | Asteraceae ⁄ Compositae |
| Genus: | Heterotheca |
| Species: | H. subaxillaris |
| Binomial name | |
| Heterotheca subaxillaris (Lam.) Britton & Rusby | |
| |
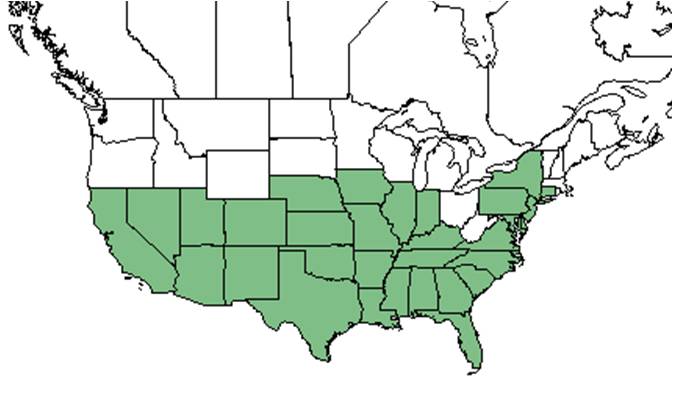
| Natural range of Heterotheca subaxillaris from USDA NRCS Plants Database. | |
Common name: Camphorweed
Contents
Taxonomic notes
Synonyms: none.[1]
Varieties: none.[1]
Description
A description of Heterotheca subaxillaris is provided in The Flora of North America.
Heterotheca subaxillaris is an annual in temperate climates and a short lived perennial in subtropical climates.[2] Leaves are simple and are alternately arranged, with one leaf per node. The leaves produce a camphor-like aroma which defers herbivores.[3] The yellow composite flowers are arranged in diffuse, paniculate corymbs. It is a C3 plant in carbon fixation. [2]
Distribution
The range of this plant occurs as north as New Jersey, south to Florida, and west to Texas and Mexico.[1]
Ecology
Habitat
In the Coastal Plain in Florida and Georgia, H. subaxillaris occurs in open sand with sea oats, pine-scrub oak sand ridges, longleaf pine-wiregrass savannas, and upper beaches. It thrives in disturbed areas and has been observed to be a ruderal species that quickly colonizes xeric habitats. It is able to compete well against other species in disturbed habitats due to the overwintering rosettes shading competing seeds. [2] It has been found in disturbed areas such as railroad bridges, powerline corridors, vacant lots, along highways, wet roadside ditches, near phosphate ponds, fire lines, disturbed coastal dunes, and cleared sand pine-evergreen oak scrub. [4] Areas in which it inhabits typically have low levels of soil nutrients and high sand temperature [2] when nitrogen levels are low, it produces an increased amount of allelochemicals and allocates more carbon to root growth. [5] Soil types include loamy sand and sand. [4] Associated species include Andropogon, Baccharis, Setaria, Cenchrus, Distichlis, Paspalum urvillei, P. notatum, Eragrostis oxylepis, Eleusine indica, Digitaria sanguinalis, Cyperus surinamensis, Ambrosia artemisiifolia, Strophostyles helvola, Solanum americanum, S. sisymbrifolium, Daubentonia drummondii, and Sesbania exaltata. [4]
Phenology
H. subaxillaris has been observed to flower March through January and fruit in September. [4][6] It is self-incompatible and depends on insects for pollination. [2] H. subaxillaris has been observed flowering at different times in different areas, probably an adaptation to a shorter growing season. [7]
Seed dispersal
Seeds are dispersed by wind. [2]
Seed bank and germination
H. subaxillaris is a heterocarpic species,[8] the achenes produced by the ray flowers lack a pappus and require a long period of dormancy.[3] The achenes produced are dimorphic and lack albumin.[2]
Germination occurs in the spring or fall; with seeds that germinate in the fall overwintering in a rosette and forming a taproot.[9] In coastal dunes, there is an absence of a persistent seed bank for H. subaxillaris; however, it has been observed to germinate from a highly disturbed seed bank. Seedlings form dense mats below the dead parent plants in late winter and early spring.[2]
Pollination
Heterotheca subaxillaris has been observed at the Archbold Biological Station to be pollinated by ground-nesting bees from the Andrenidae family (Andrena fulvipennis), sweat wasps from the Halictidae family (Agapostemon splendens, Augochloropsis metallica, A. sumptuosa, Halictus poeyi, Lasioglossum nymphalis and L. tamiamensis), leafcutting bees from the Megachilidae family (Anthidium maculifrons, Coelioxys mexicana, Megachile albitarsis, M. brevis pseudobrevis, M. inimica and M. xylocopoides), thread-waisted wasps from the Sphecidae family (Bicyrtes capnoptera, Microbembex monodonta and Tachytes validus), and wasps from the Vespidae family (Pachodynerus erynnis, Stenodynerus fundatiformis and Zethus spinipes).[10] Additionally, this species has been observed with ground-nesting bees from the Andrenidae family (Calliopsis sp., Macrotera sp., Perdita sp., Protandrena sp., and Pseudopanurgus sp.), long-tongued bees from the Apidae family (Anthophora sp., Bombus sp., Ceratina sp., Exomalopsis sp., Holcopasites sp., Nomada sp., Svastra sp. and Tetraloniella sp.), plasterer bees from the Colletidae family (Colletes sp.), sweat bees from the Halictidae family (Agapostemon sp., Dieunomia sp., Halictus sp. and Lasioglossum sp.), leafcutting bees from the Megachille family (Ashmeadiella sp., Coelioxys sp., Dianthidium sp., Heriades sp., Megachile sp. and Paranthidium sp.), thread-waisted wasps from the Sphecidae family (Sphex ichneumoneus), planthoppers from the Delphacidae family (Pissonotus delicatus), plant bugs from the Miridae family (Macrotylus amoenus), stink bugs from the Pentatomidae family (Euschistus servus), and assassin bugs from the Reduviidae family (Apiomerus crassipes and Apiomerus flaviventris).[11]
In defoliated plants, there is an increased biomass allocation to shoot growth, maintaining a balance between carbon and nutrient income similar to non defoliated plants. Defoliated plants have a slow growth rate, suggesting that even though proportionally greater allocation to the shoots occurs after defoliation, both carbon income and nutrient uptake are reduced. [12]
Conservation, cultivation, and restoration
Cultural use
Photo Gallery
References and notes
Cheplick, G.P., 2005. Patterns in the distribution of American beachgrass (Ammophila breviligulata) and the density and reproduction of annual plants on a coastal beach. Plant Ecology, 180, 57-67
Olsen, Kenneth M.. “Pollination Effectiveness and Pollinator Importance in a Population of Heterotheca Subaxillaris (asteraceae)”. Oecologia 109.1 (1997): 114–121
- ↑ 1.0 1.1 1.2 Weakley, A.S. 2015. Flora of the southern and mid-atlantic states. Working Draft of 21 May 2015. University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Lonard, Robert I., Frank W. Judd, and Richard Stalter. “Biological Flora of Coastal Dunes and Wetlands: Heterotheca Subaxillaris (J. De Lamarck) N. Britton & H. Rusby”. Journal of Coastal Research 27.6 (2011): 1052–1058.
- ↑ 3.0 3.1 [[1]]Accessed: December 15, 2015
- ↑ 4.0 4.1 4.2 4.3 Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: October 2015. Collectors: Loran C. Anderson, Robert Blaisdell, A.F. Clewell, G. Crosby, M. Darst, Dawn Doran, Geriuld Wilhelm, Mark A. Garland, Robert K. Godfrey, Bruce Hansen, JoAnn Hansen, C. Jackson, R. Kral, Robert L. Lazor, Robert J. Lemaire, D.W. Mather, Travis MacClendon, Sidney McDaniel, Richard S. Mitchell, John B. Nelson, Gwynn W. Ramsey, R.L. Redfearn Jr., Cecil R. Slaughter, H. Larry Stripling, Bian Tan, D.B. Ward. States and Counties: Florida: Bay, Calhoun, Citrus, Clay, Columbia, Escambia, Franklin, Hernando, Indian River, Jackson, Leon, Levy, Madison, Marion, Okaloosa, Pinellas, Polk, Suwannee, St. Lucie, Wakulla. Georgia: Jasper. Compiled by Tall Timbers Research Station and Land Conservancy.
- ↑ Charles A. Mihaliak, and David E. Lincoln. “Plant Biomass Partitioning and Chemical Defense: Response to Defoliation and Nitrate Limitation”. Oecologia 80.1 (1989): 122–126.
- ↑ Nelson, G. PanFlora: Plant data for the eastern United States with emphasis on the Southeastern Coastal Plains, Florida, and the Florida Panhandle. www.gilnelson.com/PanFlora/ Accessed: 12 DEC 2016
- ↑ Burk, C. John. “Rainfall Periodicity as a Major Factor in the Formation of Flowering Races of Camphorweed (heterotheca Subaxillaris)”. American Journal of Botany 53.9 (1966): 933–936.
- ↑ J. Phil Gibson and Amelia D. Tomlinson (2002). "Genetic Diversity and Mating System Comparisons between Ray and Disc Achene Seed Pools of the Heterocarpic Species Heterotheca subaxillaris (Asteraceae)." International Journal of Plant Sciences 163(6): 1025-1034
- ↑ Guertin, P. and Halvorson, W.L. 2003. Factsheet for: Heterotheca subaxillaris (Lam. )Brtit & Rusby. Tucson, Arizona: U.S. Geological Survey 25 p.
- ↑ Deyrup, M.A. and N.D. 2015. Database of observations of Hymenoptera visitations to flowers of plants on Archbold Biological Station, Florida, USA.
- ↑ Discoverlife.org [2]
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedMihaliak and Lincoln 1989