Difference between revisions of "Drosera capillaris"
m (→Description) |
HaleighJoM (talk | contribs) (→Ecology) |
||
| (25 intermediate revisions by 6 users not shown) | |||
| Line 20: | Line 20: | ||
==Taxonomic Notes== | ==Taxonomic Notes== | ||
| − | Synonym(s): | + | Synonym(s): none.<ref name="weakley">Weakley, A.S. 2015. Flora of the southern and mid-atlantic states. Working Draft of 21 May 2015. University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.</ref> |
| + | |||
| + | Varieties: none.<ref name="weakley">Weakley, A.S. 2015. Flora of the southern and mid-atlantic states. Working Draft of 21 May 2015. University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.</ref> | ||
==Description== | ==Description== | ||
<!-- Basic life history facts such as annual/perrenial, monoecious/dioecious, root morphology, seed type, etc. --> | <!-- Basic life history facts such as annual/perrenial, monoecious/dioecious, root morphology, seed type, etc. --> | ||
| − | ''D. capillaris'' is a dioecious perennial forb/herb.<ref name="USDA"/> It also is a carnivorous plant that preys upon many species of arthropods<ref name="Jennings et al 2010"/> by trapping and digesting them in their "dew." Such behavior is seen as an adaptation to overcome the nutrient poor soils with | + | ''D. capillaris'' is a dioecious perennial forb/herb that is a member of the Droseraceae family.<ref name="USDA"/> Stems are 1-2 cm long containing a rosette of leaves. Petioles are 5-10 mm long, glabrous, and dilated. Leaf blades are typically longer than the petiole, measuring 4-10 mm and cuneate. Scapes extend 4-9 cm and are gladular pubescent and bear 1-8 flowers.<ref name="Wynne 1944"/> It also is a carnivorous plant that preys upon many species of arthropods<ref name="Jennings et al 2010"/> by trapping and digesting them in their mucilaginous secretion known as "dew."<ref>Dexheimer J. (1978). Study of mucilage secretion by the cells of the digestive glands of ''Drosera capensis'' L. using staining of the plasmalemma and mucilage by phosphotungstic acid. Cytologia 43(1):45-52.</ref> Such behavior is seen as an adaptation to overcome the nutrient poor soils with additional sources of nitrogen, phosphorus, potassium and magnesium,<ref name="Adamec 2002">Adamec L. (2002). Leaf absorption of mineral nutrients in carnivorous plants stimulates root nutrient uptake. New Phytologist 155(1):89-100.</ref><ref name="Ellison 2006">Ellison A. M. (2006). Nutrient Limitation and Stoichiometry of Carnivorous Plants. Plant Biology 8(6):740-747.</ref> although nutrient absorption in the leaves have also been shown to stimulate root nutrient uptake.<ref name="Adamec 2002"/> Seeds are brown elliptic to oblong-ovate (0.4-0.5 mm long), asymmetric, and coarsely papillose-corrugated in 14-16 ridges.<ref name="Wynne 1944"/> Average maximum root depth of ''D. capillaris'' was found to be 3 cm.<ref>Brewer, J. S., et al. (2011). "Carnivory in plants as a beneficial trait in wetlands." Aquatic Botany 94: 62-70.</ref> |
| − | |||
==Distribution== | ==Distribution== | ||
| Line 31: | Line 32: | ||
==Ecology== | ==Ecology== | ||
| + | Species in the ''Drosera'' genus are carnivorous plants, where they grow in boggy and nutrient depleted habitats and have evolved to trap and digest insects to attain extra nutrients, like nitrogen, that they would not otherwise get in their environment.<ref>[[http://www.growsundews.com/sundews/sundew_drosera_information.html]] Accessed: May 3, 2019</ref> | ||
| + | |||
===Habitat=== <!--Natural communities, human disturbed habitats, topography, hydrology, soils, light, fire regime requirements for removal of competition, etc.--> | ===Habitat=== <!--Natural communities, human disturbed habitats, topography, hydrology, soils, light, fire regime requirements for removal of competition, etc.--> | ||
| − | It is an obligate wetland species<ref name="USDA"/> being found in pine savannas and other wet sandy or peaty soils.<ref name="Weakley 2015"/> | + | It is an obligate wetland species<ref name="USDA"/> being found in pine savannas and other wet sandy or peaty soils.<ref name="Weakley 2015"/> ''D. capillaris'' has been observed in various habitats including wet ditches, roadside depressions and other wet disturbed areas, exposed patches of wet sand, wet sandy clearings, wet mud of drying pond margin, wet savannas, hillside bogs, pine flatwoods, moist sandflats, and acidic sandy-clay shores. It was also seen to survive drying out sites of these habitats.<ref name= "herbarium">Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: May 2019. Collectors: Loran C. Anderson, C. R. Bell, Robert S. Blaisdell, Edwin L. Bridges, A. F. Clewell, D. S. Correll, Kathy Craddock Burks, George R. Cooley, R. A. Davidson, J. P. Gillespie, Robert K. Godfrey, Floyd Griffith, C. Jackson, S. B. Jones, R. Komarek, - Kral, Robert Kral, O. Lakela, Fred L. Lawton, Robert J. Lemaire, S. W. Leonard, Sidney McDaniel, E. C. Ogden, Steve L. Orzell, James D. Ray, Jr., P. L. Redfearn, Jr., W. D. Reese, J. D. Reynolds, Cecil R. Slaughter, Rosalind Thebaud, R. F. Thorne, L. B. Trott, Kenneth A. Wilson, and Carroll E. Wood, Jr. States and Counties: Florida: Bay, Calhoun, Columbia, Dixie, Escambia, Franklin, Gadsden, Hernando, Hillsborough, Jackson, Jefferson, Lafayette, Leon, Levy, Liberty, Madison, Nassau, Okaloosa, Orange, Osceola, Polk, Santa Rosa, Sarasota, St Johns, St Lucie, Union, Wakulla, Walton, and Washington. Alabama: Baldwin. Georgia: Brantley, Grady, and Thomas. Mississippi: Covington and Pearl River. South Carolina: Allendale. Texas: Newton.</ref> |
| + | |||
| + | Associated species: ''Drosera brevifolia'', ''Xyris'' sp., ''Taxodium ascendens'', ''Pinguicula'' sp., ''Pogonia'' sp., ''Sarracenia psittacina'', ''Sarracenia'' sp., ''Serenoa repens'', ''Utricularia subulata'', ''Utricularia cornuta'', ''Rhynchospora oligantha'', ''Eriocaulon compressum'', ''Stillingia aquatica'', ''Lachnocaulon'' sp., and ''Sphagnum'' sp.<ref name= "herbarium"/> | ||
===Phenology=== <!--Timing off flowering, fruiting, seed dispersal, and environmental triggers. Cite PanFlora website if appropriate: http://www.gilnelson.com/PanFlora/ --> | ===Phenology=== <!--Timing off flowering, fruiting, seed dispersal, and environmental triggers. Cite PanFlora website if appropriate: http://www.gilnelson.com/PanFlora/ --> | ||
| − | + | Generally, ''D. capillaris'' flowers from May until August.<ref name= "Weakley 2015"/> It has been observed flowering from March to July with peak inflorescence in April and May.<ref name="Brewer 1999"/><ref>Nelson, G. PanFlora: Plant data for the eastern United States with emphasis on the Southeastern Coastal Plains, Florida, and the Florida Panhandle. www.gilnelson.com/PanFlora/ Accessed: 6 DEC 2016</ref> The probability of a rosette flowering is primarily dependent upon its size.<ref name="Brewer 1999"/> Flowers are about 0.4 in (1 cm) in diameter with pink petals 6-7 mm long and 2-3 mm wide. Sepals are oblong-elliptic stretching 3-4 mm long and 1-2 mm wide and the flowers have 3 styles bipartite to their base.<ref name="Wynne 1944">Wynne F. E. (1944). ''Drosera'' in eastern North America. Bulletin of the Torrey Botanical Club 71(2):166-174.</ref> | |
<!--===Seed dispersal===--> | <!--===Seed dispersal===--> | ||
| Line 42: | Line 47: | ||
===Fire ecology=== <!--Fire tolerance, fire dependence, adaptive fire responses--> | ===Fire ecology=== <!--Fire tolerance, fire dependence, adaptive fire responses--> | ||
| − | Fires facilitate the occurrence of ''D. capillaris'' by eliminating or reducing competition.<ref name="Brewer 1999">Brewer J. S. (1999). Effects of fire, competition and soil disturbances on regeneration of a carnivorous plant (''Drosera capillaris''). American Midland Naturalist 141:28-42.</ref> Seedling density also increased following burns, although the growth rates of seedlings remained unaffected.<ref name="Brewer 1999"/> Growth rates are instead dictated by level of competition.<ref name="Brewer 1999"/> | + | Fires facilitate the occurrence of ''D. capillaris'' by eliminating or reducing competition.<ref name="Brewer 1999">Brewer J. S. (1999). Effects of fire, competition and soil disturbances on regeneration of a carnivorous plant (''Drosera capillaris''). American Midland Naturalist 141:28-42.</ref> Seedling density also increased following burns, although the growth rates of seedlings remained unaffected.<ref name="Brewer 1999"/> Growth rates are instead dictated by level of competition.<ref name="Brewer 1999"/> Flowering is reported to increase following fires.<ref>Hinman S. E. and Brewer J. S. (2007). Responses of two frequently-burned wet pine savannas to an extended period without fire. Journal of the Torrey Botanical Society 134(4):512-526.</ref> As well, ''D. capillaris'' responded positively to fires outside of the lightning season in December and in May.<ref name= "Brewer 2002">Brewer, J. S. (2002). Are pine-savanna plants adapted to fires outside the lightning season? [abstract]. Abstracts from the Ecological Society of America 87th Annual Meeting and the Society for Ecological Restoration 14th Annual International Conference, Tucson, AZ, Ecological Society of America, Washington D.C.</ref> |
| − | <!--===Pollination===--> | + | |
| + | <!--===Pollination===--> | ||
| − | === | + | ===Herbivory and toxicology===<!--Herbivory, granivory, insect hosting, etc.--> |
| − | Sundews are generalists, preying upon a range of arthropods including those from Diptera (true flies), Collembola (Springtails), and Formicidae (ants). Diet overlap suggests competition occurs between ''D. capillaris'' and predatory insects including wolf spiders (Lycosidae).<ref name="Jennings et al 2010">Jennings D. E., Krupa J. J., Raffel T. R., and Rohr J. R. (2010). Evidence for competition between carnivorous plants and spiders. Proceedings of the Royal Society B: Biological Sciences, doi:10.1098/rspb.2010.0465 </ref> Although a predator to many insects, the larvae of the plume moth (''Trichoptilus parvulus'') is known to consume ''D. capillaris'' by emerging from hiding at night and eating the stalked glands of the sundew.<ref name="Eisner & Shepherd 1965">Eisner T. and Shepherd J. (1965). Caterpillar feeding on a sundew plant. Science 150(3703):1608-1609</ref> Larger larvae may also consume parts of the leaf blade in addition to the gland.<ref name="Eisner & Shepherd 1965"/> Indirect effects by other organisms also influence the pink sundew. Crayfish mound excavations bury individuals of ''D. capillaris'' as they flatten out | + | Sundews are generalists, preying upon a range of arthropods including those from Diptera (true flies), Collembola (Springtails), and Formicidae (ants). Diet overlap suggests competition occurs between ''D. capillaris'' and predatory insects including wolf spiders (Lycosidae).<ref name="Jennings et al 2010">Jennings D. E., Krupa J. J., Raffel T. R., and Rohr J. R. (2010). Evidence for competition between carnivorous plants and spiders. Proceedings of the Royal Society B: Biological Sciences, doi:10.1098/rspb.2010.0465 </ref> Although a predator to many insects, the larvae of the plume moth (''Trichoptilus parvulus'') is known to consume ''D. capillaris'' by emerging from hiding at night and eating the stalked glands of the sundew.<ref name="Eisner & Shepherd 1965">Eisner T. and Shepherd J. (1965). Caterpillar feeding on a sundew plant. Science 150(3703):1608-1609</ref> Larger larvae may also consume parts of the leaf blade in addition to the gland.<ref name="Eisner & Shepherd 1965"/> Indirect effects by other organisms also influence the pink sundew. Crayfish mound excavations bury individuals of ''D. capillaris'' as they flatten out causing mortality, especially in smaller individuals.<ref name="Brewer 1999"/> |
<!--==Diseases and parasites==--> | <!--==Diseases and parasites==--> | ||
| − | ==Conservation and | + | ==Conservation, cultivation, and restoration== |
| + | ''D. capillaris'' is listed as endangered by the Maryland Department of Natural Resources, Natural Heritage Program, and listed as threatened by the Tennessee Department of Environment and Conservation, Natural Heritage Program.<ref name= "USDA"/> | ||
| − | == | + | ==Cultural use== |
==Photo Gallery== | ==Photo Gallery== | ||
<gallery widths=180px> | <gallery widths=180px> | ||
</gallery> | </gallery> | ||
==References and notes== | ==References and notes== | ||
Latest revision as of 20:06, 27 June 2022
| Drosera capillaris | |
|---|---|

| |
| Photo by John B | |
| Scientific classification | |
| Kingdom: | Plantae |
| Division: | Magnoliophyta - Flowering plants |
| Class: | Magnoliopsida - Dicots |
| Order: | Nephenthales |
| Family: | Droseraceae |
| Genus: | Drosera |
| Species: | D. capillaris |
| Binomial name | |
| Drosera capillaris Poir. | |
| |
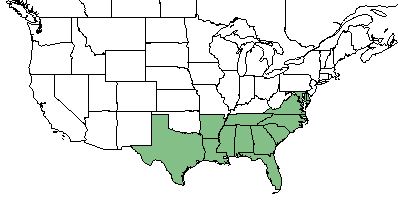
| Natural range of Drosera capillaris from USDA NRCS Plants Database. | |
Common Name(s): pink sundew[1][2]
Contents
Taxonomic Notes
Synonym(s): none.[3]
Varieties: none.[3]
Description
D. capillaris is a dioecious perennial forb/herb that is a member of the Droseraceae family.[2] Stems are 1-2 cm long containing a rosette of leaves. Petioles are 5-10 mm long, glabrous, and dilated. Leaf blades are typically longer than the petiole, measuring 4-10 mm and cuneate. Scapes extend 4-9 cm and are gladular pubescent and bear 1-8 flowers.[4] It also is a carnivorous plant that preys upon many species of arthropods[5] by trapping and digesting them in their mucilaginous secretion known as "dew."[6] Such behavior is seen as an adaptation to overcome the nutrient poor soils with additional sources of nitrogen, phosphorus, potassium and magnesium,[7][8] although nutrient absorption in the leaves have also been shown to stimulate root nutrient uptake.[7] Seeds are brown elliptic to oblong-ovate (0.4-0.5 mm long), asymmetric, and coarsely papillose-corrugated in 14-16 ridges.[4] Average maximum root depth of D. capillaris was found to be 3 cm.[9]
Distribution
Drosera capillaris is found in the southeastern United States ranging from Virginia, south to Florida, and westward to Texas. It can aslo be found in the West Indies, Mexico, and northern South America.[1]
Ecology
Species in the Drosera genus are carnivorous plants, where they grow in boggy and nutrient depleted habitats and have evolved to trap and digest insects to attain extra nutrients, like nitrogen, that they would not otherwise get in their environment.[10]
Habitat
It is an obligate wetland species[2] being found in pine savannas and other wet sandy or peaty soils.[1] D. capillaris has been observed in various habitats including wet ditches, roadside depressions and other wet disturbed areas, exposed patches of wet sand, wet sandy clearings, wet mud of drying pond margin, wet savannas, hillside bogs, pine flatwoods, moist sandflats, and acidic sandy-clay shores. It was also seen to survive drying out sites of these habitats.[11]
Associated species: Drosera brevifolia, Xyris sp., Taxodium ascendens, Pinguicula sp., Pogonia sp., Sarracenia psittacina, Sarracenia sp., Serenoa repens, Utricularia subulata, Utricularia cornuta, Rhynchospora oligantha, Eriocaulon compressum, Stillingia aquatica, Lachnocaulon sp., and Sphagnum sp.[11]
Phenology
Generally, D. capillaris flowers from May until August.[1] It has been observed flowering from March to July with peak inflorescence in April and May.[12][13] The probability of a rosette flowering is primarily dependent upon its size.[12] Flowers are about 0.4 in (1 cm) in diameter with pink petals 6-7 mm long and 2-3 mm wide. Sepals are oblong-elliptic stretching 3-4 mm long and 1-2 mm wide and the flowers have 3 styles bipartite to their base.[4]
Seed bank and germination
D. capillaris seeds are reported to be abundant in the seed banks of disturbed and undisturbed long-leaf pine habitat.[14] Emergence of seedlings typically occurs between early winter and late spring.[12]
Fire ecology
Fires facilitate the occurrence of D. capillaris by eliminating or reducing competition.[12] Seedling density also increased following burns, although the growth rates of seedlings remained unaffected.[12] Growth rates are instead dictated by level of competition.[12] Flowering is reported to increase following fires.[15] As well, D. capillaris responded positively to fires outside of the lightning season in December and in May.[16]
Herbivory and toxicology
Sundews are generalists, preying upon a range of arthropods including those from Diptera (true flies), Collembola (Springtails), and Formicidae (ants). Diet overlap suggests competition occurs between D. capillaris and predatory insects including wolf spiders (Lycosidae).[5] Although a predator to many insects, the larvae of the plume moth (Trichoptilus parvulus) is known to consume D. capillaris by emerging from hiding at night and eating the stalked glands of the sundew.[17] Larger larvae may also consume parts of the leaf blade in addition to the gland.[17] Indirect effects by other organisms also influence the pink sundew. Crayfish mound excavations bury individuals of D. capillaris as they flatten out causing mortality, especially in smaller individuals.[12]
Conservation, cultivation, and restoration
D. capillaris is listed as endangered by the Maryland Department of Natural Resources, Natural Heritage Program, and listed as threatened by the Tennessee Department of Environment and Conservation, Natural Heritage Program.[2]
Cultural use
Photo Gallery
References and notes
- ↑ 1.0 1.1 1.2 1.3 Weakley A. S.(2015). Flora of the Southern and Mid-Atlantic States. Chapel Hill, NC: University of North Carolina Herbarium.
- ↑ 2.0 2.1 2.2 2.3 USDA, NRCS. (2016). The PLANTS Database (http://plants.usda.gov, 30 November 2017). National Plant Data Team, Greensboro, NC 27401-4901 USA.
- ↑ 3.0 3.1 Weakley, A.S. 2015. Flora of the southern and mid-atlantic states. Working Draft of 21 May 2015. University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
- ↑ 4.0 4.1 4.2 Wynne F. E. (1944). Drosera in eastern North America. Bulletin of the Torrey Botanical Club 71(2):166-174.
- ↑ 5.0 5.1 Jennings D. E., Krupa J. J., Raffel T. R., and Rohr J. R. (2010). Evidence for competition between carnivorous plants and spiders. Proceedings of the Royal Society B: Biological Sciences, doi:10.1098/rspb.2010.0465
- ↑ Dexheimer J. (1978). Study of mucilage secretion by the cells of the digestive glands of Drosera capensis L. using staining of the plasmalemma and mucilage by phosphotungstic acid. Cytologia 43(1):45-52.
- ↑ 7.0 7.1 Adamec L. (2002). Leaf absorption of mineral nutrients in carnivorous plants stimulates root nutrient uptake. New Phytologist 155(1):89-100.
- ↑ Ellison A. M. (2006). Nutrient Limitation and Stoichiometry of Carnivorous Plants. Plant Biology 8(6):740-747.
- ↑ Brewer, J. S., et al. (2011). "Carnivory in plants as a beneficial trait in wetlands." Aquatic Botany 94: 62-70.
- ↑ [[1]] Accessed: May 3, 2019
- ↑ 11.0 11.1 Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: May 2019. Collectors: Loran C. Anderson, C. R. Bell, Robert S. Blaisdell, Edwin L. Bridges, A. F. Clewell, D. S. Correll, Kathy Craddock Burks, George R. Cooley, R. A. Davidson, J. P. Gillespie, Robert K. Godfrey, Floyd Griffith, C. Jackson, S. B. Jones, R. Komarek, - Kral, Robert Kral, O. Lakela, Fred L. Lawton, Robert J. Lemaire, S. W. Leonard, Sidney McDaniel, E. C. Ogden, Steve L. Orzell, James D. Ray, Jr., P. L. Redfearn, Jr., W. D. Reese, J. D. Reynolds, Cecil R. Slaughter, Rosalind Thebaud, R. F. Thorne, L. B. Trott, Kenneth A. Wilson, and Carroll E. Wood, Jr. States and Counties: Florida: Bay, Calhoun, Columbia, Dixie, Escambia, Franklin, Gadsden, Hernando, Hillsborough, Jackson, Jefferson, Lafayette, Leon, Levy, Liberty, Madison, Nassau, Okaloosa, Orange, Osceola, Polk, Santa Rosa, Sarasota, St Johns, St Lucie, Union, Wakulla, Walton, and Washington. Alabama: Baldwin. Georgia: Brantley, Grady, and Thomas. Mississippi: Covington and Pearl River. South Carolina: Allendale. Texas: Newton.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 Brewer J. S. (1999). Effects of fire, competition and soil disturbances on regeneration of a carnivorous plant (Drosera capillaris). American Midland Naturalist 141:28-42.
- ↑ Nelson, G. PanFlora: Plant data for the eastern United States with emphasis on the Southeastern Coastal Plains, Florida, and the Florida Panhandle. www.gilnelson.com/PanFlora/ Accessed: 6 DEC 2016
- ↑ Cohen S., Braham R., and Sanchez F. (2004). Seed bank viability in disturbed long-leaf pine sites. Restoration Ecology 12(4):503-515.
- ↑ Hinman S. E. and Brewer J. S. (2007). Responses of two frequently-burned wet pine savannas to an extended period without fire. Journal of the Torrey Botanical Society 134(4):512-526.
- ↑ Brewer, J. S. (2002). Are pine-savanna plants adapted to fires outside the lightning season? [abstract]. Abstracts from the Ecological Society of America 87th Annual Meeting and the Society for Ecological Restoration 14th Annual International Conference, Tucson, AZ, Ecological Society of America, Washington D.C.
- ↑ 17.0 17.1 Eisner T. and Shepherd J. (1965). Caterpillar feeding on a sundew plant. Science 150(3703):1608-1609