Difference between revisions of "Gelsemium sempervirens"
Lsandstrum (talk | contribs) |
Lsandstrum (talk | contribs) |
||
| Line 31: | Line 31: | ||
==Ecology== | ==Ecology== | ||
===Habitat=== <!--Natural communities, human disturbed habitats, topography, hydrology, soils, light, fire regime requirements for removal of competition, etc.--> | ===Habitat=== <!--Natural communities, human disturbed habitats, topography, hydrology, soils, light, fire regime requirements for removal of competition, etc.--> | ||
| − | This species is found in a large range of habitats, including swamp forests to even dry uplands as well as thickets, and it is also commonly an ornamental plant otherwise. It commonly climbs to the tops of trees.<ref name= "Weakley"/> As well, it is frequently found in abandoned fields, and can grow into a mound of tangled stems if left untouched.<ref name= "lady bird">[[https://www.wildflower.org/plants/search.php?search_field=&newsearch=true]] Lady Bird Johnson Wildflower Center. Accessed: May 17, 2019</ref> It can grow on a wild range of soils, including sandy, sandy loam, medium loam, clay loam, and clay. It grows best on well-drained, moist, and humus-rich soil, and is very adaptable to varying pH levels.<ref name= "lady bird"/> ''Gelsemium sempervirens'' has been observed along a hardwood slope and bottomland forest with other vines, in open areas of mixed pinelands, in mesic woodlands, creek shores, a rich hammock, floodplain forests, swampy flatwoods, and a variety of disturbed habitats.<ref name= "herbarium">Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: May 2019. Collectors: Loran C. Anderson, Kathy Craddock Burks, Chris Cooksey, Patricia Elliot, Robert K. Godfrey, C. Jackson, Gary R. Knight, R. Komarek, Robert Kral, Richard S. Mitchell, J. Nelson, George M. Riegler, L. B. Trott, D. B. Ward, and S. S. Ward. States and Counties: Florida: Calhoun, De Soto, Franklin, Gulf, Jackson, Jefferson, Leon, Liberty, Madison, Marion, Okaloosa, Pasco, Taylor, Volusia, Wakulla, Walton, and Washington. Georgia: Grady and Thomas.</ref> Frequency has been recorded to increase with thinning or clearcutting the overstory.<ref>Brockway, D. G. and C. E. Lewis (2003). "Influence of deer, cattle grazing and timber harvest on plant species diversity in a longleaf pine bluestem ecosystem." Forest Ecology and Management 175: 49-69.</ref> ''G. sempervirens'' responds positively to agricultural-based soil disturbance in South Carolina coastal plains communities.<ref>Brudvig, L.A., J.L. Orrock, E.I. Damschen, C.D. Collins, P.G. Hahn, W.B. Mattingly, J.W. Veldman, and J.L. Walker. (2014). Land-Use History and Contemporary Management Inform an Ecological Reference Model for Longleaf Pine Woodland Understory Plant Communities. PLoS ONE 9(1): e86604.</ref> It also responds positively to soil disturbance by heavy silvilculture in North Carolina.<ref>Cohen, S., R. Braham, and F. Sanchez. (2004). Seed Bank Viability in Disturbed Longleaf Pine Sites. Restoration Ecology 12(4):503-515.</ref> | + | This species is found in a large range of habitats, including swamp forests to even dry uplands as well as thickets, and it is also commonly an ornamental plant otherwise. It commonly climbs to the tops of trees.<ref name= "Weakley"/> As well, it is frequently found in abandoned fields, and can grow into a mound of tangled stems if left untouched.<ref name= "lady bird">[[https://www.wildflower.org/plants/search.php?search_field=&newsearch=true]] Lady Bird Johnson Wildflower Center. Accessed: May 17, 2019</ref> It can grow on a wild range of soils, including sandy, sandy loam, medium loam, clay loam, and clay. It grows best on well-drained, moist, and humus-rich soil, and is very adaptable to varying pH levels.<ref name= "lady bird"/> ''Gelsemium sempervirens'' has been observed along a hardwood slope and bottomland forest with other vines, in open areas of mixed pinelands, in mesic woodlands, creek shores, a rich hammock, floodplain forests, swampy flatwoods, and a variety of disturbed habitats.<ref name= "herbarium">Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: May 2019. Collectors: Loran C. Anderson, Kathy Craddock Burks, Chris Cooksey, Patricia Elliot, Robert K. Godfrey, C. Jackson, Gary R. Knight, R. Komarek, Robert Kral, Richard S. Mitchell, J. Nelson, George M. Riegler, L. B. Trott, D. B. Ward, and S. S. Ward. States and Counties: Florida: Calhoun, De Soto, Franklin, Gulf, Jackson, Jefferson, Leon, Liberty, Madison, Marion, Okaloosa, Pasco, Taylor, Volusia, Wakulla, Walton, and Washington. Georgia: Grady and Thomas.</ref> Frequency has been recorded to increase with thinning or clearcutting the overstory.<ref>Brockway, D. G. and C. E. Lewis (2003). "Influence of deer, cattle grazing and timber harvest on plant species diversity in a longleaf pine bluestem ecosystem." Forest Ecology and Management 175: 49-69.</ref> ''G. sempervirens'' responds positively to agricultural-based soil disturbance in South Carolina coastal plains communities.<ref>Brudvig, L.A., J.L. Orrock, E.I. Damschen, C.D. Collins, P.G. Hahn, W.B. Mattingly, J.W. Veldman, and J.L. Walker. (2014). Land-Use History and Contemporary Management Inform an Ecological Reference Model for Longleaf Pine Woodland Understory Plant Communities. PLoS ONE 9(1): e86604.</ref> It also responds positively to soil disturbance by heavy silvilculture in North Carolina.<ref>Cohen, S., R. Braham, and F. Sanchez. (2004). Seed Bank Viability in Disturbed Longleaf Pine Sites. Restoration Ecology 12(4):503-515.</ref> ''G. sempervirens'' respond relatively positively, or not at all, to heavy silvilculture in North Florida. <ref>Conde, L.F., B.F. Swindel, and J.E. Smith. (1986). Five Years of Vegetation Changes Following Conversion of Pine Flatwoods to ''Pinus elliottii'' Plantations. Forest Ecology and Management 15(4):295-300.</ref> |
Associated species include ''Acer rubrum'', ''Celtis'' sp., ''Morus'' sp., ''Platanus'' sp., ''Populus'' sp., ''Quercus'' sp., ''Ulmus'' sp., ''Liriodendron tulipifera'', ''Lyonia'' sp., ''Gordonia'' sp., ''Vitis'' sp., and other vines.<ref name= "herbarium"/> | Associated species include ''Acer rubrum'', ''Celtis'' sp., ''Morus'' sp., ''Platanus'' sp., ''Populus'' sp., ''Quercus'' sp., ''Ulmus'' sp., ''Liriodendron tulipifera'', ''Lyonia'' sp., ''Gordonia'' sp., ''Vitis'' sp., and other vines.<ref name= "herbarium"/> | ||
Revision as of 14:17, 10 July 2019
| Gelsemium sempervirens | |
|---|---|

| |
| Photo by John R. Gwaltney, Southeastern Flora.com | |
| Scientific classification | |
| Kingdom: | Plantae |
| Division: | Magnoliophyta - Flowering plants |
| Class: | Magnoliopsida - Dicotyledons |
| Order: | Gentianales |
| Family: | Loganiaceae |
| Genus: | Gelsemium |
| Species: | G. sempervirens |
| Binomial name | |
| Gelsemium sempervirens (L.) W.T. Aiton | |
| |
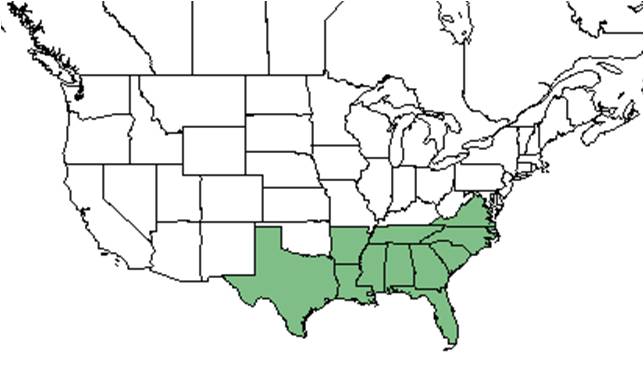
| Natural range of Gelsemium sempervirens from USDA NRCS Plants Database. | |
Common names: Evening trumpetflower; Yellow jessamine; Carolina jessamine
Contents
Taxonomic notes
Description
"High climbing or trailing vines, twining upward from left to right. Leaves evergreen, opposite, lanceolate to elliptic, 3-7 cm long, 1-2.5 cm wide, acute to acuminate, entire, base rounded to cuneate; petioles 2-7 mm long. Flowers actinomorphic, heterostylic, axillary, solitary or in cymes; pedicels short, bracteate. Sepals 5, separate to base, lanceolate,, 3-5 mm long, 1-2 mm wide; corolla 5-lobed, yellow, 2-3.8 cm long, the tube gradually flaring upward, lobes 7-10 mm long, spreading; stamens 5, attached to lower part of corolla tube, anthers sagittate; pistil 1, slender styled, 2-cleft, each divided again and appearing 4-cleft. Capsule compressed; seeds many, dull brown, the body roughly papillose." [1]
"Leaf base cuneate to rounded. Flowers very fragrant, usually solitary, rarely in 2-3 flowered cymes. Speals obtuse to subacute. Capsule oblong, 1.4-2 cm long, 0.8-1.2 cm broad, abruptly rounded to a beaked apex; seeds 0.7-1 cm long, membranously winged apically."[1]
Distribution
This species is generally found along the southeastern coastal plain from Virginia and Tennessee south to Florida and west to Texas and Arkansas.[2] More specifically, it can be found from Virginia, southeastern Tennessee, and Arkansas south to central peninsular Florida and eastern Texas. It is also disjunct in Mexico and Guatemala.[3]
Ecology
Habitat
This species is found in a large range of habitats, including swamp forests to even dry uplands as well as thickets, and it is also commonly an ornamental plant otherwise. It commonly climbs to the tops of trees.[3] As well, it is frequently found in abandoned fields, and can grow into a mound of tangled stems if left untouched.[4] It can grow on a wild range of soils, including sandy, sandy loam, medium loam, clay loam, and clay. It grows best on well-drained, moist, and humus-rich soil, and is very adaptable to varying pH levels.[4] Gelsemium sempervirens has been observed along a hardwood slope and bottomland forest with other vines, in open areas of mixed pinelands, in mesic woodlands, creek shores, a rich hammock, floodplain forests, swampy flatwoods, and a variety of disturbed habitats.[5] Frequency has been recorded to increase with thinning or clearcutting the overstory.[6] G. sempervirens responds positively to agricultural-based soil disturbance in South Carolina coastal plains communities.[7] It also responds positively to soil disturbance by heavy silvilculture in North Carolina.[8] G. sempervirens respond relatively positively, or not at all, to heavy silvilculture in North Florida. [9]
Associated species include Acer rubrum, Celtis sp., Morus sp., Platanus sp., Populus sp., Quercus sp., Ulmus sp., Liriodendron tulipifera, Lyonia sp., Gordonia sp., Vitis sp., and other vines.[5]
Phenology
Generally, G. sempervirens flowers from February to early May, and fruits from September to November.[3] It has been observed to flower from January to April with peak inflorescence in February.[10] It has also been observed fruiting in February and July.[5] Although it usually blooms briefly in the early spring, it can begin flowering as early as December and also bloom again in the early fall. Greatest inflorescence is in full sun.[4]
Seed dispersal
This species is thought to be dispersed by gravity. [11]
Seed bank and germination
Although commonly found as herbaceous vegetation, G. sempervirens was not found in the seed bank of multiple studies.[12][13][14]
Fire ecology
Gelsemium sempevirens has been observed growing in areas that are fire-disturbed.[15] However, it has been shown to either decrease in frequency not change much after a fire disturbance.[16][17] Winter and spring burn regiments benefit G. sempervirens more than summer burn regiments.[18] It has been found to proliferate in fire-excluded areas.[19]
Pollination
The following Hymenoptera families and species were observed visiting flowers of Gelsemium sempervirens at Archbold Biological Station: [20]
Apidae: Bombus griseocollis, B. impatiens, Habropoda laboriosa
This species has also been observed to be pollinated by Xylocopa virginica krombeini in the Hymenoptera order.[21]
Use by animals
It consists of approximately 5-10% of the diet for large mammals and various terrestrial birds.[22] It has been recorded to be eaten by white-tailed deer[23]; it is highly preferred by deer since they forage it year round.[24] G. sempervirens has also been recorded to be eaten by the Florida marsh rabbit.[25] The highest amount of protein, calcium, and phosphorus production of this species is in the spring, and fiber content stays mostly consistent throughout the year.[24] The leaves, roots, and flowers are poisonous for humans and livestock, and can be lethal.[4] However, it has been recorded to be eaten by cattle.[26] The nectar can also be toxic for honeybees if too much is consumed, and the nectar and honey produced from this nectar may also be toxic for humans. The sap from this species can cause dermatitis in humans. Otherwise, the flowers attract hummingbirds, native bees, and spicebush swallowtail butterflies. It is highly deer resistant.[4] Medicinally, the root of this plant is considered a febrifuge, an anti-spasmodic, a nervine, an alternative, and an emmenagogue.[27] As well, the root and flowers are narcotic, and their effluvia can cause stupor. A tincture of the root can be used for rheumatism that is in frictions.[28] In previous times, it was in reasonably constant demand and of commercial importance.[29]
Diseases and parasites
Due to it being tenacious and adaptable overall, G. sempervirens does not have any serious insect or disease problems.[4] However it is a host plant for false spider mites, including Brevipalpus californicus and B. obovatus.[30]
Conservation and management
It is considered a species of special concern by the Tennessee Department of Environment and Conservation, Natural Heritage Program.[2] For general management, it should be pruned in the early spring to maintain its shape and keep it from taking over.[4]
Cultivation and restoration
Photo Gallery
References and notes
- ↑ 1.0 1.1 Radford, Albert E., Harry E. Ahles, and C. Ritchie Bell. Manual of the Vascular Flora of the Carolinas. 1964, 1968. The University of North Carolina Press. Print.
- ↑ 2.0 2.1 USDA, NRCS. (2016). The PLANTS Database (http://plants.usda.gov, 17 May 2019). National Plant Data Team, Greensboro, NC 27401-4901 USA.
- ↑ 3.0 3.1 3.2 Weakley, A. S. (2015). Flora of the Southern and Mid-Atlantic States. Chapel Hill, NC, University of North Carolina Herbarium.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 [[1]] Lady Bird Johnson Wildflower Center. Accessed: May 17, 2019
- ↑ 5.0 5.1 5.2 Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: May 2019. Collectors: Loran C. Anderson, Kathy Craddock Burks, Chris Cooksey, Patricia Elliot, Robert K. Godfrey, C. Jackson, Gary R. Knight, R. Komarek, Robert Kral, Richard S. Mitchell, J. Nelson, George M. Riegler, L. B. Trott, D. B. Ward, and S. S. Ward. States and Counties: Florida: Calhoun, De Soto, Franklin, Gulf, Jackson, Jefferson, Leon, Liberty, Madison, Marion, Okaloosa, Pasco, Taylor, Volusia, Wakulla, Walton, and Washington. Georgia: Grady and Thomas.
- ↑ Brockway, D. G. and C. E. Lewis (2003). "Influence of deer, cattle grazing and timber harvest on plant species diversity in a longleaf pine bluestem ecosystem." Forest Ecology and Management 175: 49-69.
- ↑ Brudvig, L.A., J.L. Orrock, E.I. Damschen, C.D. Collins, P.G. Hahn, W.B. Mattingly, J.W. Veldman, and J.L. Walker. (2014). Land-Use History and Contemporary Management Inform an Ecological Reference Model for Longleaf Pine Woodland Understory Plant Communities. PLoS ONE 9(1): e86604.
- ↑ Cohen, S., R. Braham, and F. Sanchez. (2004). Seed Bank Viability in Disturbed Longleaf Pine Sites. Restoration Ecology 12(4):503-515.
- ↑ Conde, L.F., B.F. Swindel, and J.E. Smith. (1986). Five Years of Vegetation Changes Following Conversion of Pine Flatwoods to Pinus elliottii Plantations. Forest Ecology and Management 15(4):295-300.
- ↑ Nelson, G. PanFlora: Plant data for the eastern United States with emphasis on the Southeastern Coastal Plains, Florida, and the Florida Panhandle. www.gilnelson.com/PanFlora/ Accessed: 9 DEC 2016
- ↑ Kirkman, L. Katherine. Unpublished database of seed dispersal mode of plants found in Coastal Plain longleaf pine-grasslands of the Jones Ecological Research Center, Georgia.
- ↑ Andreu, M. G., et al. (2009). "Can managers bank on seed banks when restoring Pinus taeda L. plantations in Southwest Georgia?" Restoration Ecology 17: 586-596.
- ↑ Cohen, S., et al. (2004). "Seed bank viability in disturbed longleaf pine sites." Restoration Ecology 12: 503-515.
- ↑ Collins, B. and G. Wein (1995). "Seed bank and vegetation of a constructed reservoir." Wetlands 15(4): 374-385.
- ↑ Glitzenstein, J. S., et al. (2012). "Fire-frequency effects on vegetation in north Florida pinelands: Another look at the long-term Stoddard Fire Research Plots at Tall Timbers Research Station." Forest Ecology and Management 264: 197-209.
- ↑ Haywood, J. D., et al. (1995). Responses of understory vegetation on highly erosive Louisiana soils to prescribed burning in May. Asheville, NC, USDA Forest Service, Research Note SO-383: 8.
- ↑ Lay, D. W. (1967). "Browse palatability and the effects of prescribed burning in southern pine forests." Journal of Forestry 65: 826-828.
- ↑ Kush, J. S., et al. (2000). Understory plant community response to season of burn in natural longleaf pine forests. Proceedings 21st Tall Timbers Fire Ecology Conference. Fire and forest ecology: innovative silviculture & vegetation management, Tallahassee, FL, Tall Timbers Research, Inc.
- ↑ Clewell, A. F. (2014). "Forest development 44 years after fire exclusion in formerly annually burned oldfield pine woodland, Florida." Castanea 79: 147-167.
- ↑ Deyrup, M.A. and N.D. 2015. Database of observations of Hymenoptera visitations to flowers of plants on Archbold Biological Station, Florida, USA.
- ↑ Deyrup, M. J. E., and Beth Norden (2002). "The diversity and floral hosts of bees at the Archbold Biological Station, Florida (Hymenoptera: Apoidea)." Insecta mundi 16(1-3).
- ↑ Miller, J.H., and K.V. Miller. 1999. Forest plants of the southeast and their wildlife uses. Southern Weed Science Society.
- ↑ Atwood, E. L. (1941). "White-tailed deer foods of the United States." The Journal of Wildlife Management 5(3): 314-332.
- ↑ 24.0 24.1 Blair, R. M. and E. A. Epps (1969). Seasonal distribution of nutrients in plants of seven browse species in Louisiana. Research Paper SO-51. New Orleans, LA, USDA Forest Service.
- ↑ Blair, W. F. (1936). "The Florida marsh rabbit." Journal of Mammalogy 17(3): 197-207.
- ↑ Thill, R. E. (1984). "Deer and cattle diets on Louisiana pine-hardwood sites." The Journal of Wildlife Management 48(3): 788-798.
- ↑ Nickell, J. M. (1911). J.M.Nickell's botanical ready reference : especially designed for druggists and physicians : containing all of the botanical drugs known up to the present time, giving their medical properties, and all of their botanical, common, pharmacopoeal and German common (in German) names. Chicago, IL, Murray & Nickell MFG. Co.
- ↑ Rafinesque, C. S. (1828). Medical flora; or Manual of the medical botany of the United States of North America.
- ↑ Sievers, A. F. (1930). American medicinal plants of commercial importance. Washington, USDA.
- ↑ Childers, C. C., et al. (2003). "Host plants of Brevipalpus californicus, B. obovatus, and B. phoenicis (Acari: Tenuipalpidae) and their potential involvement in the spread of viral diseases vectored by these mites." Experimental & Applied Acarology 30: 29-105.