Difference between revisions of "Mimosa quadrivalvis"
| Line 32: | Line 32: | ||
===Habitat=== <!--Natural communities, human disturbed habitats, topography, hydrology, soils, light, fire regime requirements for removal of competition, etc.--> | ===Habitat=== <!--Natural communities, human disturbed habitats, topography, hydrology, soils, light, fire regime requirements for removal of competition, etc.--> | ||
| − | This species has been found in open pinewoods, oak-scrub woodlands, prairies, limestone glades, deciduous forests, turkey oak-pinelands, savannas, well drained ridges, ungrazed native grasslands, arroyos, and bare chalk areas (FSU Herbarium). It is commonly found in pine sandhill environments ( | + | This species has been found in open pinewoods, oak-scrub woodlands, prairies, limestone glades, deciduous forests, turkey oak-pinelands, savannas, well drained ridges, ungrazed native grasslands, arroyos, and bare chalk areas (FSU Herbarium). It is commonly found in pine sandhill environments. <ref name=dow> Downer, M. R. (2012). Plant species richness and species area relationships in a Florida sandhill community. Integrative Biology. Ann Arbor, MI, University of South Florida. M.S.: 52. </ref> Occurs in areas that have sandy loam, peat, gravel, and/or clay loam that is dry, loose, or moist (Miller et al 1999, FSU Herbarium). ''Mimosa quadrivalvis'' is predominately in native groundcover with a statistical affinity in upland pinelands of South Georgia (Ostertag and Robertson 2007). This species can be found growing in open to semi-shaded areas (FSU Herbarium). Associated species include ''Pinus palutris, Quercus laevis, Muhlenbergia, Aristida longespica, A. oligantha, Juniperus, Chamaecyparis, Sabal, Magnolia, Acer, Illicium, Salix, Asclepias curtissii, Palafoxia, Paroncyhia, Quercus myrtifolia, Q. geminata,'' and ''Serenoa repens'' (FSU Herbarium). |
===Phenology=== <!--Timing off flowering, fruiting, seed dispersal, and environmental triggers. Cite PanFlora website if appropriate: http://www.gilnelson.com/PanFlora/ --> | ===Phenology=== <!--Timing off flowering, fruiting, seed dispersal, and environmental triggers. Cite PanFlora website if appropriate: http://www.gilnelson.com/PanFlora/ --> | ||
Revision as of 19:48, 12 April 2016
| Mimosa quadrivalvis | |
|---|---|

| |
| Photo take by Michelle M. Smith | |
| Scientific classification | |
| Kingdom: | Plantae |
| Division: | Magnoliophyta - Flowering plants |
| Class: | Magnoliopsida – Dicotyledons |
| Order: | Fabales |
| Family: | Fabaceae ⁄ Leguminosae |
| Genus: | Mimosa |
| Species: | M. quadrivalvis |
| Binomial name | |
| Mimosa quadrivalvis L. | |
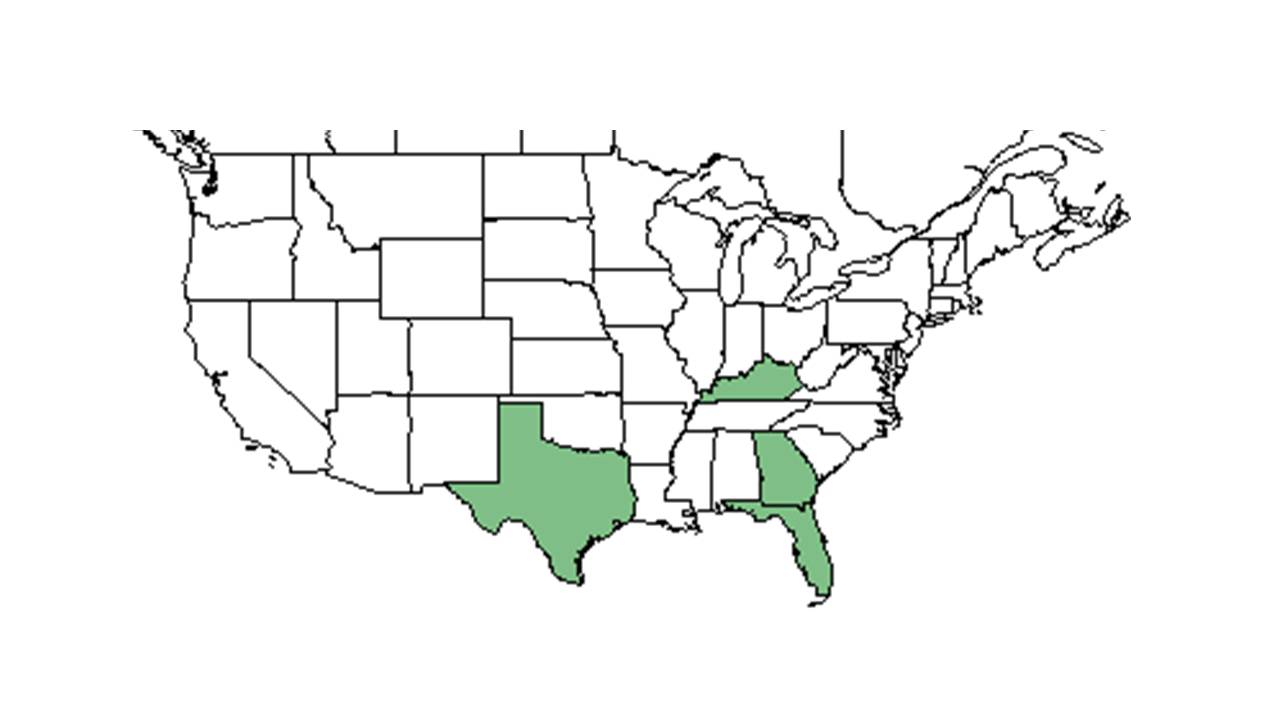
| |
| Natural range of Mimosa quadrivalvis from USDA NRCS Plants Database. | |
Common name: fourvalve mimosa
Contents
Taxonomic notes
Synonyms: Leptoglottis quadrivalvis (L.) Britton & Rose; Mimosa quadrivalvis var. quadrivalvis; Schrankia quadrivalvis (L.) Merr.; The Plant List.org
Description
This species is frequent where it is found and has a sprawling behavior (FSU Herbarium). It is a vining plant that creeps and crawls as well (FSU Herbarium).
Distribution
Ecology
In a study comparing N2 fixation potential in nine legume species occurring in longleaf pine-wiregrass ecosystems, M. quadrivalvis showed clear superiority in growing a large nodule mass to support the high N2 fixation activity.[1] Aboveground N concentration was the greatest for M. quadrivalis species.[1] N2 fixation potential for M. quadrivalvis does not differ between shaded and unshaded environments.[1] The high potential for N2 fixation makes M. quadrivalvis a candidate species for contributing to the N economy in the restoration of longleaf pine ecosystems.[1]
Habitat
This species has been found in open pinewoods, oak-scrub woodlands, prairies, limestone glades, deciduous forests, turkey oak-pinelands, savannas, well drained ridges, ungrazed native grasslands, arroyos, and bare chalk areas (FSU Herbarium). It is commonly found in pine sandhill environments. [2] Occurs in areas that have sandy loam, peat, gravel, and/or clay loam that is dry, loose, or moist (Miller et al 1999, FSU Herbarium). Mimosa quadrivalvis is predominately in native groundcover with a statistical affinity in upland pinelands of South Georgia (Ostertag and Robertson 2007). This species can be found growing in open to semi-shaded areas (FSU Herbarium). Associated species include Pinus palutris, Quercus laevis, Muhlenbergia, Aristida longespica, A. oligantha, Juniperus, Chamaecyparis, Sabal, Magnolia, Acer, Illicium, Salix, Asclepias curtissii, Palafoxia, Paroncyhia, Quercus myrtifolia, Q. geminata, and Serenoa repens (FSU Herbarium).
Phenology
This species has been observed flowering in February as well as April through September and fruiting from April through September (FSU Herbarium).
Seed dispersal
According to Kay Kirkman, a plant ecologist, this species disperses by gravity. [3] [4]
Seed bank and germination
Fire ecology
This species has been found in areas that are burned (FSU Herbarium).
Pollination
The following Hymenoptera families and species were observed visiting flowers of Mimosa quadrivalvi at Archbold Biological Station (Deyrup 2015):
Colletidae: Colletes distinctus
Halictidae: Agapostemon splendens, Augochlorella aurata, Augochloropsis metallica, A. sumptuosa, Lasioglossum miniatulus, L. nymphalis, L. placidensis
Sphecidae: Prionyx thomae
Use by animals
Deyrup observed these bees, Agapostemon splendens, Augochlorella aurata, Augochloropsis sumptuosa, Dialictus miniatulus, D. placidensis, Anthidiellum perplexum on M. quadrivalvis [5]
Diseases and parasites
Conservation and Management
Cultivation and restoration
Photo Gallery
References and notes
Cathey, S. E., L. R. Boring, et al. (2010). "Assessment of N2 fixation capability of native legumes from the longleaf pine-wiregrass ecosystem." Environmental and Experimental Botany 67: 444-450.
Deyrup, M. J. E., and Beth Norden (2002). "The diversity and floral hosts of bees at the Archbold Biological Station, Florida (Hymenoptera: Apoidea)." Insecta mundi 16(1-3).
Deyrup, M.A. and N.D. 2015. Database of observations of Hymenoptera visitations to flowers of plants on Archbold Biological Station, Florida, USA.
Downer, M. R. (2012). Plant species richness and species area relationships in a Florida sandhill community. Integrative Biology. Ann Arbor, MI, University of South Florida. M.S.: 52.
Florida State University Robert K. Godfrey Herbarium database. URL: http://herbarium.bio.fsu.edu. Last accessed: June 2014. Collectors: Edwin L. Tyson, J. Dwyer, Kurt Blum, Robert L. Lazor, Loran C. Anderson, J, Craddock Burks, Grady W. Reinert, R.C. Phillips, Gary R. Knight, Norlan C. Henderson, R. Kral, A.F. Clewell, Robert K. Godfrey, William Reese, Paul Redfearn, J.P. Gillespie, Nancy Caswell, Richard S. Mitchell, Patricia Elliot, Gwynn W. Ramsey, D. B. Ward, S. S. Ward, D.S. Correll, Robert J. Lemaire, L.S. Beard, R.O. Vail, W.B. Fox, Edward E. Terrell, S.B. Jones, Sidney McDaniel, V.L. Cory, Brunelle Moon, Cecil R. Slaughter, Robert R. Simons, Angus Gholson, Walter S. Judd, Bob Simons, Tom Morris, William Lindsey, Elmer C. Prichard, L.J. Brass, O. Lakela, John W. Thieret, Gerardo Garcia, Anastacio Bernal, Natalio Castillo, Guillermo Perez, Nick Lopez, W. L. McCart, D. S. Correll, I. M. Johnston, Duane Isely, F. L. Lewton, H. R. Reed, L. S. Beard, and R O Vail. States and Counties: Florida: Calhoun, Citrus, Duval, Franklin, Gadsden, Highlands, Indian River, Jackson, Lake, Leon, Levy, Liberty, Madison, Marion, Nassau, Osceola, Pinellas, Polk, Suwannee, Taylor, Wakulla, Walton, and Volusia. Georgia: Seminole, and Thomas. Arkansas: Clark and Washington. North Carolina: Robeson and Rockingham. Alabama: Pickens and Sumter. Louisiana: Acadia. Texas: Andrews,Aransas, Callahan, Comal, Dallas, Denton, Dimmit, Ft. McKanepp, Haskell, Leon, Sutton, Taylor, Van Zandt, and Zapata. Kansas: Franklin. Missouri: Bates and Greene. Countries: Panama.
Maza-Villalobos, S., C. Lemus-Herrera, et al. (2011). "Successional trends in soil seed banks of abandoned pastures of a Neotropical dry region." Journal of Tropical Ecology 27: 35-49
Miller, J. H., R. S. Boyd, et al. (1999). "Floristic diversity, stand structure, and composition 11 years after herbicide site preparation." Canadian Journal of Forest Research 29: 1073-1083.
Ostertag, T.E., and K.M. Robertson. 2007. A comparison of native versus old-field vegetation in upland pinelands managed with frequent fire, South Georgia, USA. Pages 109–120 in R.E. Masters and K.E.M. Galley (eds.). Proceedings of the 23rd Tall Timbers Fire Ecology Conference: Fire in Grassland and Shrubland Ecosystems.
- ↑ 1.0 1.1 1.2 1.3 Cathey, S. E., L. R. Boring, et al. (2010). "Assessment of N2 fixation capability of native legumes from the longleaf pine-wiregrass ecosystem." Environmental and Experimental Botany 67: 444-450.
- ↑ Downer, M. R. (2012). Plant species richness and species area relationships in a Florida sandhill community. Integrative Biology. Ann Arbor, MI, University of South Florida. M.S.: 52.
- ↑ Kay Kirkman, unpublished data, 2015.
- ↑ Maza-Villalobos, S., C. Lemus-Herrera, et al. (2011). "Successional trends in soil seed banks of abandoned pastures of a Neotropical dry region." Journal of Tropical Ecology 27: 35-49.
- ↑ Deyrup, M. J. E., and Beth Norden (2002). "The diversity and floral hosts of bees at the Archbold Biological Station, Florida (Hymenoptera: Apoidea)." Insecta mundi 16(1-3).